Page 203 - Applied Process Design For Chemical And Petrochemical Plants Volume III
P. 203
66131_Ludwig_CH10F 5/30/2001 4:35 PM Page 166
166 Applied Process Design for Chemical and Petrochemical Plants
T w temperature of tube heating surface, °R A well-recognized and often-used equation for determin-
T s saturation temperature of liquid, °R ing a reasonable, even if preliminary, nucleate boiling coef-
B L coefficient of Figure 10-99 ficient is represented by the McNelly equation for boiling
h fg latent heat of evaporation, Btu/lb outside of tubes:
0.3 0.6 2 0.425
D o G v c f f P a
The value of this relation is that it serves as a maximum h b
1c L G v 2c d c d c d (10-140)
f k L L
limit that may be expected from a designed unit when com-
paring design Q/A versus Equation 10-139.
where
Mikic and Rohsenow present another boiling correla-
86
Q L V L
tion for pool boiling, which includes the effects of the heat- G v c d c d (10-141)
ing surface characteristics. A nf v A nf v
It is important to design at values of temperature differ-
2
ence below the critical value separating nucleate and film h b boiling side film coefficient, Btu/hr-ft -°F
surface condition factor:
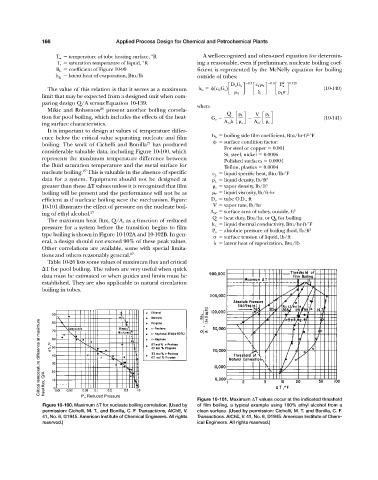
boiling. The work of Cichelli and Bonilla has produced
27
For steel or copper 0.001
considerable valuable data, including Figure 10-100, which
St. steel, nickel 0.0006
represents the maximum temperature difference between
Polished surfaces 0.0004
the fluid saturation temperature and the metal surface for Teflon, plastics 0.0004
27
nucleate boiling. This is valuable in the absence of specific c L liquid specific heat, Btu/lb-°F
data for a system. Equipment should not be designed at L liquid density, lb/ft 3
greater than these T values unless it is recognized that film v vapor density, lb/ft 3
boiling will be present and the performance will not be as f liquid viscosity, lb/ft-hr
efficient as if nucleate boiling were the mechanism. Figure D o tube O.D., ft
10-101 illustrates the effect of pressure on the nucleate boil- V vapor rate, lb/hr 2
ing of ethyl alcohol. 27 A nf surface area of tubes, outside, ft
Q heat duty, Btu/hr, or Q b for boiling
The maximum heat flux, Q/A, as a function of reduced
k L liquid thermal conductivity, Btu/hr-ft-°F
pressure for a system before the transition begins to film
P a absolute pressure of boiling fluid, lb/ft 2
type boiling is shown in Figure 10-102A and 10-102B. In gen-
surface tension of liquid, lb/ft
eral, a design should not exceed 90% of these peak values. latent heat of vaporization, Btu/lb
Other correlations are available, some with special limita-
tions and others reasonably general. 97
Table 10-26 lists some values of maximum flux and critical
T for pool boiling. The values are very useful when quick
data must be estimated or when guides and limits must be
established. They are also applicable to natural circulation
boiling in tubes.
Critical temperature difference at maximum
heat flux, Q/A
P r , Reduced Pressure
Figure 10-101. Maximum T values occur at the indicated threshold
Figure 10-100. Maximum T for nucleate boiling correlation. (Used by of film boiling, a typical example using 100% ethyl alcohol from a
permission: Cichelli, M. T., and Bonilla, C. F. Transactions, AlChE, V. clean surface. (Used by permission: Cichelli, M. T. and Bonilla, C. F.
41, No. 6, ©1945. American Institute of Chemical Engineers. All rights Transactions. AlChE, V. 41, No. 6, ©1945. American Institute of Chem-
reserved.) ical Engineers. All rights reserved.)