Page 186 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 186
THE HEAVIER PNICTOGENS
166
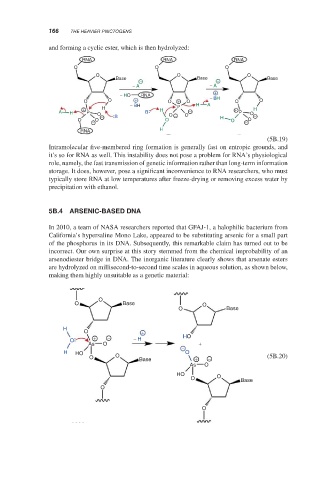
and forming a cyclic ester, which is then hydrolyzed:
RNA RNA RNA
O O O
O O O
Base Base Base
− −
− A − A
+
− HO RNA
O O + O + O − BH O O
− BH P H A
+ H H + H
A H P O B O − O − P O
O O − B O H O O −
− −
H
RNA
(5B.19)
Intramolecular five-membered ring formation is generally fast on entropic grounds, and
it’s so for RNA as well. This instability does not pose a problem for RNA’s physiological
role, namely, the fast transmission of genetic information rather than long-term information
storage. It does, however, pose a significant inconvenience to RNA researchers, who must
typically store RNA at low temperatures after freeze-drying or removing excess water by
precipitation with ethanol.
ARSENIC-BASED DNA
5B.4
In 2010, a team of NASA researchers reported that GFAJ-1, a halophilic bacterium from
California’s hypersaline Mono Lake, appeared to be substituting arsenic for a small part
of the phosphorus in its DNA. Subsequently, this remarkable claim has turned out to be
incorrect. Our own surprise at this story stemmed from the chemical improbability of an
arsenodiester bridge in DNA. The inorganic literature clearly shows that arsenate esters
are hydrolyzed on millisecond-to-second time scales in aqueous solution, as shown below,
making them highly unsuitable as a genetic material:
O
O Base O
O Base
H
O +
+ − HO
O − H
As O +
−
H HO O
O O Base + − (5B.20)
As O
HO O
O Base
O
O