Page 185 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 185
5B.3 PHOSPHORUS IN BIOLOGY: WHY NATURE CHOSE PHOSPHATE 165
pyrophosphate linkages. These are the famous high energy “bonds” of ATP. Hydrolysis of
these bonds releases a great deal of energy, for much the same reason that hydrolysis of
P O 10 does. In phosphorylation, ATP transfers a phosphoryl group to a nucleophile such
4
as an alcohol (glucose in the example above), forming a high energy C–O–phosphoryl
linkage; in brief, this is the basis of ATP’s role as the cell’s energy currency.
Many proteins are inactive in their pristine form and must be “switched on” for activ-
ity. Phosphorylation, catalyzed by kinases, is very often that switch. The reverse reaction,
dephosphorylation, then is the “off switch” that shuts down the protein’s activity; enzymes
called phosphatases catalyze this process. Reversible phosphorylation is thus an important
regulatory mechanism in cells. Phosphorylation typically occurs on amino acids with nucle-
ophilic side chains, namely, serine, threonine, tyrosine, and histidine and, in prokaryotes,
even lysine and arginine. Phosphorylation greatly increases the hydrophilicity of a given
part of the protein, which may lead to large conformational changes that are necessary for
activity. Alternatively, phosphorylation may turn an alcoholic OH group, one of organic
chemistry’s notoriously poor leaving groups, into phosphate, a much better one.
Another class of phosphate derivatives of paramount importance in biology consists of
phosphate diesters or phosphodiesters (to be distinguished from organic diphosphates or
pyrophosphates, which are key intermediates in steroid biosynthesis), in which two of the
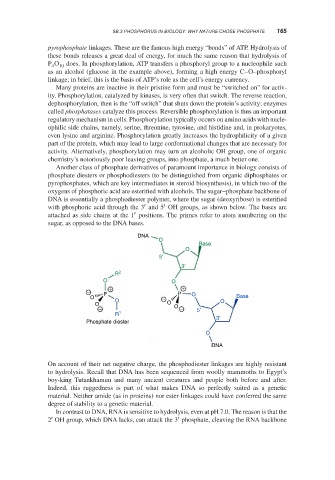
oxygens of phosphoric acid are esterified with alcohols. The sugar–phosphate backbone of
DNA is essentially a phosphodiester polymer, where the sugar (deoxyribose) is esterified
′ ′
with phosphoric acid through the 3 and 5 OH groups, as shown below. The bases are
′
attached as side chains at the 1 positions. The primes refer to atom numbering on the
sugar, as opposed to the DNA bases.
DNA
O
Base
O
5′
3′
R 2
O O
− P + P + O
O O − O Base
O O O
− − 5′
R 1 3′
Phosphate diester
O
DNA
On account of their net negative charge, the phosphodiester linkages are highly resistant
to hydrolysis. Recall that DNA has been sequenced from woolly mammoths to Egypt’s
boy-king Tutankhamun and many ancient creatures and people both before and after.
Indeed, this ruggedness is part of what makes DNA so perfectly suited as a genetic
material. Neither amide (as in proteins) nor ester linkages could have conferred the same
degree of stability to a genetic material.
In contrast to DNA, RNA is sensitive to hydrolysis, even at pH 7.0. The reason is that the
′ ′
2 OH group, which DNA lacks, can attack the 3 phosphate, cleaving the RNA backbone