Page 180 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 180
THE HEAVIER PNICTOGENS
160
OH
− − +
HO O HO O HO P
H H H O OH
P P −
O + O HO + O
O HO + + H
+ O + − O +
H − P O P O − P + O P OH OH O
O P O P + OH +
O + O O O P P
− − O OH
O − O O P
O O
H O − O +
H
H
OH O
+ OH +
− P O P OH 3 H 3 PO 4
O P
O + O
− O
(5B.6)
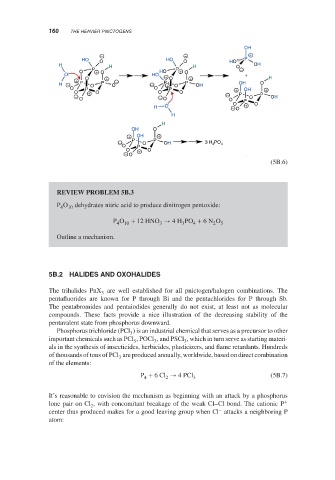
REVIEW PROBLEM 5B.3
P O 10 dehydrates nitric acid to produce dinitrogen pentoxide:
4
P O + 12 HNO → 4H PO + 6N O 5
10
2
4
3
3
4
Outline a mechanism.
5B.2 HALIDES AND OXOHALIDES
The trihalides PnX are well established for all pnictogen/halogen combinations. The
3
pentafluorides are known for P through Bi and the pentachlorides for P through Sb.
The pentabromides and pentaiodides generally do not exist, at least not as molecular
compounds. These facts provide a nice illustration of the decreasing stability of the
pentavalent state from phosphorus downward.
Phosphorus trichloride (PCl ) is an industrial chemical that serves as a precursor to other
3
important chemicals such as PCl ,POCl , and PSCl , which in turn serve as starting materi-
5 3 3
als in the synthesis of insecticides, herbicides, plasticizers, and flame retardants. Hundreds
of thousands of tons of PCl are produced annually, worldwide, based on direct combination
3
of the elements:
P + 6Cl → 4PCl 3 (5B.7)
4
2
It’s reasonable to envision the mechanism as beginning with an attack by a phosphorus
+
lone pair on Cl , with concomitant breakage of the weak Cl–Cl bond. The cationic P
2
–
center thus produced makes for a good leaving group when Cl attacks a neighboring P
atom: