Page 184 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 184
THE HEAVIER PNICTOGENS
164
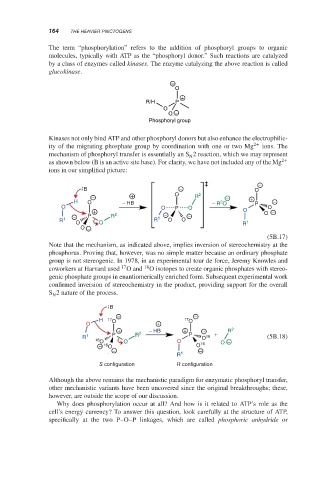
The term “phosphorylation” refers to the addition of phosphoryl groups to organic
molecules, typically with ATP as the “phosphoryl donor.” Such reactions are catalyzed
by a class of enzymes called kinases. The enzyme catalyzing the above reaction is called
glucokinase.
−
O
+
R/H P
O
O −
Phosphoryl group
Kinases not only bind ATP and other phosphoryl donors but also enhance the electrophilic-
ity of the migrating phosphate group by coordination with one or two Mg 2+ ions. The
mechanism of phosphoryl transfer is essentially an S 2 reaction, which we may represent
N
as shown below (B is an active site base). For clarity, we have not included any of the Mg 2+
ions in our simplified picture:
−
B − O
− + O R 2 − +
H O − HB − R O P −
2
O O P O O
+ O O −
− P R 2 − −
R 1 R 1 O O
O O R 1
O −
(5B.17)
Note that the mechanism, as indicated above, implies inversion of stereochemistry at the
phosphorus. Proving that, however, was no simple matter because an ordinary phosphate
group is not stereogenic. In 1978, in an experimental tour de force, Jeremy Knowles and
coworkers at Harvard used 17 O and 18 O isotopes to create organic phosphates with stereo-
genic phosphate groups in enantiomerically enriched form. Subsequent experimental work
confirmed inversion of stereochemistry in the product, providing support for the overall
S 2 nature of the process.
N
B
− −
H 17 17 O
O O +
+ − HB + − R 2
R 1 P R 2 P O 18 + (5B.18)
18 O O O O −
− 16 O O 16
− −
R 1
S configuration R configuration
Although the above remains the mechanistic paradigm for enzymatic phosphoryl transfer,
other mechanistic variants have been uncovered since the original breakthroughs; these,
however, are outside the scope of our discussion.
Why does phosphorylation occur at all? And how is it related to ATP’s role as the
cell’s energy currency? To answer this question, look carefully at the structure of ATP,
specifically at the two P–O–P linkages, which are called phosphoric anhydride or