Page 189 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 189
5B.5 ARSENIC TOXICITY AND BIOMETHYLATION 169
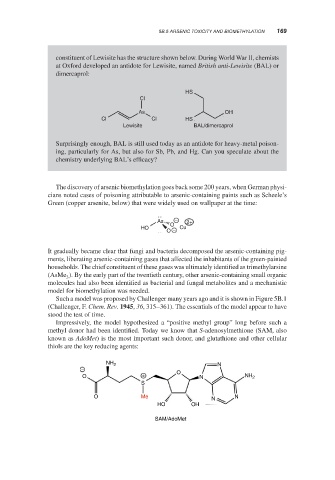
constituent of Lewisite has the structure shown below. During World War II, chemists
at Oxford developed an antidote for Lewisite, named British anti-Lewisite (BAL) or
dimercaprol:
HS
Cl
As OH
Cl Cl HS
Lewisite BAL/dimercaprol
Surprisingly enough, BAL is still used today as an antidote for heavy-metal poison-
ing, particularly for As, but also for Sb, Pb, and Hg. Can you speculate about the
chemistry underlying BAL’s efficacy?
The discovery of arsenic biomethylation goes back some 200 years, when German physi-
cians noted cases of poisoning attributable to arsenic-containing paints such as Scheele’s
Green (copper arsenite, below) that were widely used on wallpaper at the time:
As − 2+
O
HO Cu
O −
It gradually became clear that fungi and bacteria decomposed the arsenic-containing pig-
ments, liberating arsenic-containing gases that affected the inhabitants of the green-painted
households. The chief constituent of these gases was ultimately identified as trimethylarsine
(AsMe ). By the early part of the twentieth century, other arsenic-containing small organic
3
molecules had also been identified as bacterial and fungal metabolites and a mechanistic
model for biomethylation was needed.
Such a model was proposed by Challenger many years ago and it is shown in Figure 5B.1
(Challenger, F. Chem. Rev. 1945, 36, 315–361). The essentials of the model appear to have
stood the test of time.
Impressively, the model hypothesized a “positive methyl group” long before such a
methyl donor had been identified. Today we know that S-adenosylmethione (SAM, also
known as AdoMet) is the most important such donor, and glutathione and other cellular
thiols are the key reducing agents:
NH 2 N
− O
O + N NH 2
S
O Me N N
HO OH
SAM/AdoMet