Page 193 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 193
5B.7 DISPROPORTIONATION OF HYPOPHOSPHOROUS ACID 173
REVIEW PROBLEM 5B.11
Aqueous hypophosphorous acid can be obtained by simple neutralization of the reac-
tion mixture at the end of the above reaction (5B.30) with strong acid:
− +
H PO + H → H PO
2 2 3 2
An alternative synthesis of hypophosphorous acid involves oxidation of phosphine
with iodine in water:
− +
PH + 2I + 2H O → H PO + 4I + 4H
3
2
2
3
2
Suggest a mechanism for this last reaction.
5B.7 DISPROPORTIONATION OF HYPOPHOSPHOROUS ACID
The thermal decomposition of hypophosphorous acid provides a couple of perfect oppor-
tunities for sharpening our arrow-pushing skills:
2H PO → PH + H PO (5B.31)
3 2 3 3 4
3H PO → PH + 2H PO 3 (5B.32)
3
3
2
3
Which one of the two reactions predominates depends on the temperature of the reaction.
Mechanistically, both reactions seem rather impenetrable, but the trick is once again to
calmly follow the guidelines we espoused in Section 1.23. Look carefully at the products
and think about what bonds have been broken and formed in the course of the reaction. In
essence, two or three phosphorus atoms have come together and reshuffled the hydrogen
and oxygen ligands among them. For each of the above reactions, one phosphorus atom has
ended up with no oxygen at all—as PH . What kind of process would account for that?
3
Protonation of the phosphorus atom in H PO and dissociation of water as leaving
3
2
groups would not account for the generation of phosphine, for the simple reason that the P in
H PO is cationic and does not have a lone pair that can be protonated. The only alternative
3 2
–
that comes to mind involves hydride (H ) transfer from one phosphorus to another. It’s an
uncommon process in inorganic chemistry, but it’s common enough for carbocations (see
Section 1.18) so it’s not really esoteric. Let’s consider the first of the above two reactions
in light of this idea.
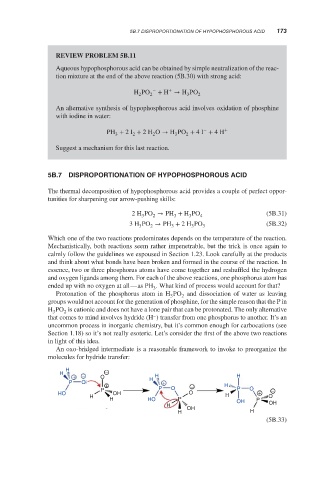
An oxo-bridged intermediate is a reasonable framework to invoke to preorganize the
molecules for hydride transfer:
H
H −
+ − O H H H
P O +
+ − H
P P O P O −
HO OH O H +
H O
H HO P OH P
H OH OH
H H
(5B.33)