Page 198 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 198
THE HEAVIER PNICTOGENS
178
Molecular mass of desired product
% Atom economy= × 100%
Molecular mass of all reactants
In other words, on a mass basis, a substantial fraction of the starting materials does not end
up as part of the desired product, but is discarded as waste. In principle, a catalytic version
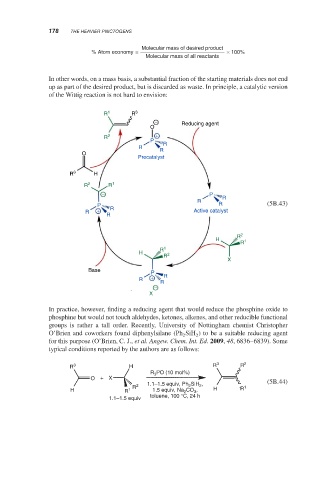
of the Wittig reaction is not hard to envision:
R 1 R 3
− Reducing agent
O
R 2 +
P
R
R
O R
Precatalyst
R 3 H
R 2 R 1
− P
R
R
P R (5B.43)
R + R Active catalyst
R
R 2
H 1
R
R 1
H 2
R
X
Base P
R + R R
−
X
In practice, however, finding a reducing agent that would reduce the phosphine oxide to
phosphine but would not touch aldehydes, ketones, alkenes, and other reducible functional
groups is rather a tall order. Recently, University of Nottingham chemist Christopher
O’Brien and coworkers found diphenylsilane (Ph SiH ) to be a suitable reducing agent
2
2
for this purpose (O’Brien, C. J., et al. Angew. Chem. Int. Ed. 2009, 48, 6836–6839). Some
typical conditions reported by the authors are as follows:
R 3 H R 3 R 2
PO (10 mol%)
R 3
O + X (5B.44)
R 2 1.1–1.5 equiv, Ph 2 SiH 2 , 1
H R 1 1.5 equiv, Na 2 CO , H R
3
toluene, 100 °C, 24 h
1.1–1.5 equiv