Page 196 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 196
THE HEAVIER PNICTOGENS
176
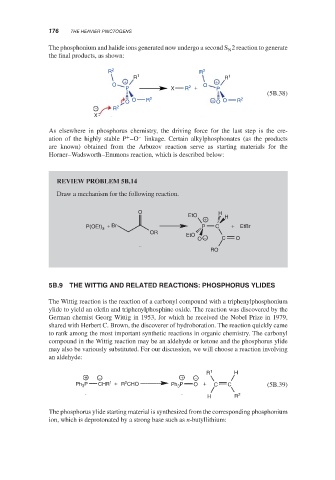
The phosphonium and halide ions generated now undergo a second S 2 reaction to generate
N
the final products, as shown:
R 2 R 2
R 1 R 1
+ +
O O
P X R 2 + P
(5B.38)
O O R 2 − O O R 2
− R 2
X
As elsewhere in phosphorus chemistry, the driving force for the last step is the cre-
+
–
ation of the highly stable P –O linkage. Certain alkylphosphonates (as the products
are known) obtained from the Arbuzov reaction serve as starting materials for the
Horner–Wadsworth–Emmons reaction, which is described below:
REVIEW PROBLEM 5B.14
Draw a mechanism for the following reaction.
O H
EtO H
+
P(OEt) + Br P C + EtBr
3
OR
EtO
O − C O
RO
5B.9 THE WITTIG AND RELATED REACTIONS: PHOSPHORUS YLIDES
The Wittig reaction is the reaction of a carbonyl compound with a triphenylphosphonium
ylide to yield an olefin and triphenylphosphine oxide. The reaction was discovered by the
German chemist Georg Wittig in 1953, for which he received the Nobel Prize in 1979,
shared with Herbert C. Brown, the discoverer of hydroboration. The reaction quickly came
to rank among the most important synthetic reactions in organic chemistry. The carbonyl
compound in the Wittig reaction may be an aldehyde or ketone and the phosphorus ylide
may also be variously substituted. For our discussion, we will choose a reaction involving
an aldehyde:
R 1 H
+ − + −
2
1
Ph P CHR + R CHO Ph 3 P O + C C (5B.39)
3
H R 2
The phosphorus ylide starting material is synthesized from the corresponding phosphonium
ion, which is deprotonated by a strong base such as n-butyllithium: