Page 200 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 200
THE HEAVIER PNICTOGENS
180
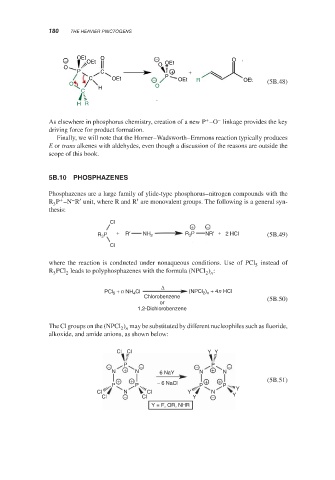
− OEt OEt O − OEt O
O O
P C + +
C OEt − P OEt R OEt
O O (5B.48)
H
C
H R
+ –
As elsewhere in phosphorus chemistry, creation of a new P –O linkage provides the key
driving force for product formation.
Finally, we will note that the Horner–Wadsworth–Emmons reaction typically produces
E or trans alkenes with aldehydes, even though a discussion of the reasons are outside the
scope of this book.
5B.10 PHOSPHAZENES
Phosphazenes are a large family of ylide-type phosphorus–nitrogen compounds with the
+ – ′ ′
R P –N R unit, where R and R are monovalent groups. The following is a general syn-
3
thesis:
Cl
+ −
+ R′ NR′ +
R P NH 2 R 3 P 2 HCl (5B.49)
3
Cl
where the reaction is conducted under nonaqueous conditions. Use of PCl instead of
5
R PCl leads to polyphosphazenes with the formula (NPCl ) :
3 2 2 n
Δ
) + 4n HCl
PCl 5 + n NH 4 Cl (NPCl 2 n
Chlorobenzene (5B.50)
or
1,2-Dichlorobenzene
The Cl groups on the (NPCl ) may be substituted by different nucleophiles such as fluoride,
2 n
alkoxide, and amide anions, as shown below:
Cl Cl YY
− P − − P −
N + N 6 NaY N + N
+ + + + (5B.51)
P P − 6 NaCl P P
Cl N Cl Y N Y
Cl − Cl Y − Y
Y = F, OR, NHR