Page 202 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 202
THE HEAVIER PNICTOGENS
182
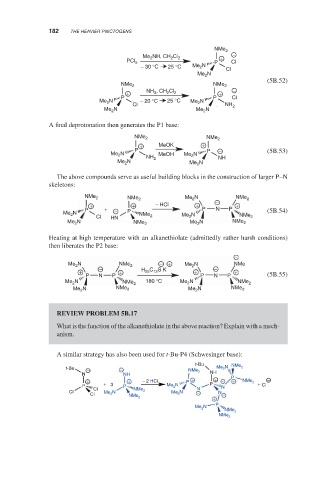
NMe 2
−
Me 2 NH, CH 2 Cl 2 +
PCl 5 P Cl
− 30 °C 25 °C Me 2 N
Cl
Me N
2
(5B.52)
NMe 2 NMe 2
+ NH 3 , CH 2 Cl 2 + −
P P Cl
N − 20 °C 25 °C Me N
Me 2 2
Cl NH 2
Me 2 N Me N
2
A final deprotonation then generates the P1 base:
NMe 2 NMe 2
+ MeOK +
P P − (5B.53)
Me 2 N MeOH Me 2 N
NH 2 NH
Me 2 N Me 2 N
The above compounds serve as useful building blocks in the construction of larger P–N
skeletons:
NMe 2 NMe 2 Me 2 N NMe 2
+ + − HCl + − +
P + − P P N P (5B.54)
Me 2 N NMe N NMe
Cl HN 2 Me 2 2
Me 2 N NMe 2 Me 2 N NMe 2
Heating at high temperature with an alkanethiolate (admittedly rather harsh conditions)
then liberates the P2 base:
−
Me N NMe 2 − + Me 2 N NMe
2
+ − + H 25 C 12 S K + − +
P N P P N P (5B.55)
Me 2 N NMe 2 180 °C Me N NMe 2
2
Me N NMe 2 Me 2 N NMe 2
2
REVIEW PROBLEM 5B.17
What is the function of the alkanethiolate in the above reaction? Explain with a mech-
anism.
A similar strategy has also been used for t-Bu-P4 (Schwesinger base):
t-Bu
t-Bu − − NMe 2 Me 2 N NMe 2
NN NH NH P
+ + − 2 HCl P + + − + NMe 2 −
P + 3 P Me 2 N P N + Cl
Cl N
Cl Me 2 N NMe 2 Me 2 N − N
Cl −
+
NMe 2
P
Me N
2
NMe 2
NMe 2