Page 206 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 206
THE HEAVIER PNICTOGENS
186
R 1 R 1
R 2 O OR R 2 O OR
C C − +
C P C P
C OR 3 C OR
R 3 O OR R O OR
R 4 R 4
R 1
R 2 O OR R 2 R 1 O OR
C + C + (5B.66)
C P + C P
−
C OR C − OR
R 3 O OR R 3 R 4 O OR
R 4
O OR OR
+ − CO 2
C P P
− OR OR
O OR OR
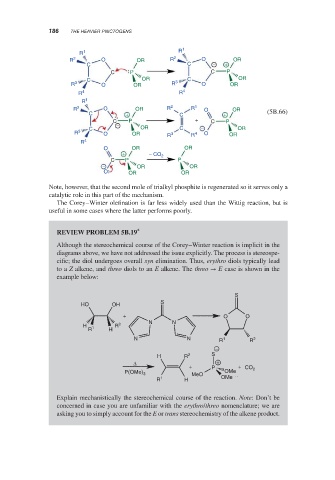
Note, however, that the second mole of trialkyl phosphite is regenerated so it serves only a
catalytic role in this part of the mechanism.
The Corey–Winter olefination is far less widely used than the Wittig reaction, but is
useful in some cases where the latter performs poorly.
REVIEW PROBLEM 5B.19 *
Although the stereochemical course of the Corey–Winter reaction is implicit in the
diagrams above, we have not addressed the issue explicitly. The process is stereospe-
cific; the diol undergoes overall syn elimination. Thus, erythro diols typically lead
to a Z alkene, and threo diols to an E alkene. The threo → E case is shown in the
example below:
S
S
HO OH
+ O O
N N
H 1 R 2
R H
N N R 1 R 2
−
H R 2 S
Δ +
+ P + CO 2
P(OMe) 3 MeO OMe
R 1 H OMe
Explain mechanistically the stereochemical course of the reaction. Note: Don’t be
concerned in case you are unfamiliar with the erythro/threo nomenclature; we are
asking you to simply account for the E or trans stereochemistry of the alkene product.