Page 209 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 209
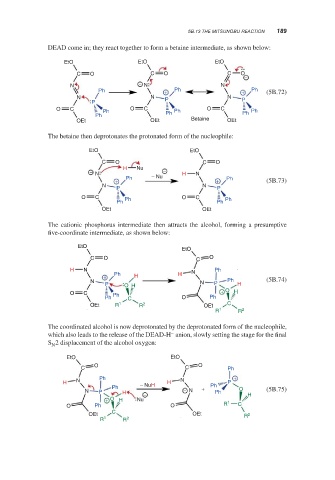
5B.13 THE MITSUNOBU REACTION 189
DEAD come in; they react together to form a betaine intermediate, as shown below:
EtO EtO EtO
C O C O C O
−
N − N N
Ph + Ph + Ph (5B.72)
N N P N P
P
O C Ph O C Ph O C Ph
Ph Ph Ph
OEt OEt Betaine OEt
The betaine then deprotonates the protonated form of the nucleophile:
EtO EtO
C O C O
H Nu
− N − Nu − H N
+ Ph + Ph (5B.73)
N N
P P
O C Ph O C
Ph Ph Ph
OEt OEt
The cationic phosphorus intermediate then attracts the alcohol, forming a presumptive
five-coordinate intermediate, as shown below:
EtO
EtO
C O C O
H N N Ph
+ Ph H H
N N Ph (5B.74)
P O H P H
O C Ph + O H
Ph C O Ph
OEt R 1 R 2 OEt C
R 1 R 2
The coordinated alcohol is now deprotonated by the deprotonated form of the nucleophile,
–
which also leads to the release of the DEAD-H anion, slowly setting the stage for the final
S 2 displacement of the alcohol oxygen:
N
EtO EtO
O O
C C Ph
Ph +
N N
H − NuH H P
Ph + Ph O
N P H − − N Ph H (5B.75)
+ O H Nu 1
O Ph O R C
C
OEt OEt R 2
R 1 R 2