Page 207 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 207
5B.12 TRIPHENYLPHOSPHINE-MEDIATED HALOGENATIONS 187
5B.12 TRIPHENYLPHOSPHINE-MEDIATED HALOGENATIONS
Triphenylphosphine is a versatile reagent in organic synthesis. In addition to the applica-
tions discussed above, it is also used in a number of halogenation protocols for converting
alcohols to alkyl halides. The Appel reaction, shown below for a primary alcohol, is used
to prepare both alkyl chlorides and bromides:
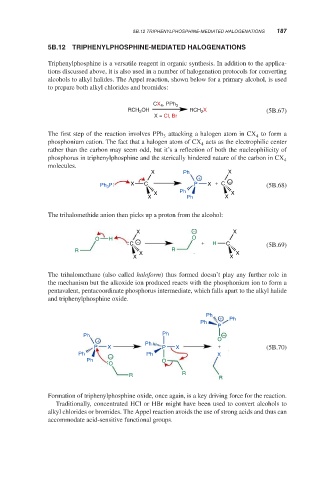
CX 4 , PPh 3
RCH 2 OH RCH 2 X (5B.67)
X = Cl, Br
The first step of the reaction involves PPh attacking a halogen atom in CX to form a
4
3
phosphonium cation. The fact that a halogen atom of CX acts as the electrophilic center
4
rather than the carbon may seem odd, but it’s a reflection of both the nucleophilicity of
phosphorus in triphenylphosphine and the sterically hindered nature of the carbon in CX 4
molecules.
X Ph X
+ −
Ph P X C P X + C (5B.68)
3
X Ph X
X Ph X
The trihalomethide anion then picks up a proton from the alcohol:
X − X
O H O
C − + H C (5B.69)
R R
X X
X X
The trihalomethane (also called haloform) thus formed doesn’t play any further role in
the mechanism but the alkoxide ion produced reacts with the phosphonium ion to form a
pentavalent, pentacoordinate phosphorus intermediate, which falls apart to the alkyl halide
and triphenylphosphine oxide.
Ph +
Ph Ph
P
Ph Ph −
+ O
P X Ph P X + (5B.70)
Ph − Ph X
Ph O
O
R R
R
Formation of triphenylphosphine oxide, once again, is a key driving force for the reaction.
Traditionally, concentrated HCl or HBr might have been used to convert alcohols to
alkyl chlorides or bromides. The Appel reaction avoids the use of strong acids and thus can
accommodate acid-sensitive functional groups.