Page 210 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 210
THE HEAVIER PNICTOGENS
190
–
We, however, need the nucleophile in its anionic form first; the DEAD-H anion serves as
the base for this purpose:
EtO EtO
O O
C C
N − N
H − Nu H (5B.76)
− N H Nu N H
O O
OEt OEt
We are now set for the grand finale—the clean stereospecific S 2 displacement of tri-
N
phenylphosphine oxide:
Ph
+
P Ph H
Ph O Nu
Ph + +
H P + R 1 C (5B.77)
Ph −
R 1 C − Ph O 2
Nu R
R 2
The value of the Mitsunobu reaction derives from the mild conditions used and the
stereospecificity of the process. It is one of the most important means for inverting a
stereogenic center in natural product synthesis. It does, however, have a few serious draw-
backs that discourage its use as a larger-scale industrial process. The Mitsunobu reaction
is very “un-green.” Not only is the triphenylphosphine “wasted” from an atom-economy
point of view, but there are also significant problems associated with DEAD, including
high toxicity, risk of explosion, and the difficulty of removing the hydrazine waste. In
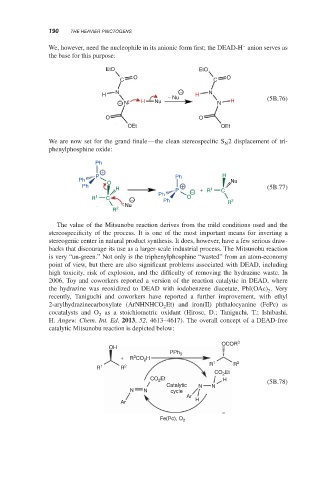
2006, Toy and coworkers reported a version of the reaction catalytic in DEAD, where
the hydrazine was reoxidized to DEAD with iodobenzene diacetate, PhI(OAc) .Very
2
recently, Taniguchi and coworkers have reported a further improvement, with ethyl
2-arylhydrazinecarboxylate (ArNHNHCO Et) and iron(II) phthalocyanine (FePc) as
2
cocatalysts and O as a stoichiometric oxidant (Hirose, D.; Taniguchi, T.; Ishibashi,
2
H. Angew. Chem. Int. Ed. 2013, 52, 4613–4617). The overall concept of a DEAD-free
catalytic Mitsunobu reaction is depicted below:
OCOR 3
OH
PPh 3
+ R CO H
3
2
R 1 R 2
R 1 R 2
CO Et
2
Et
CO 2 H (5B.78)
Catalytic N N
N N cycle
Ar
Ar H
Fe(Pc), O 2