Page 205 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 205
5B.11 THE COREY–WINTER OLEFINATION 185
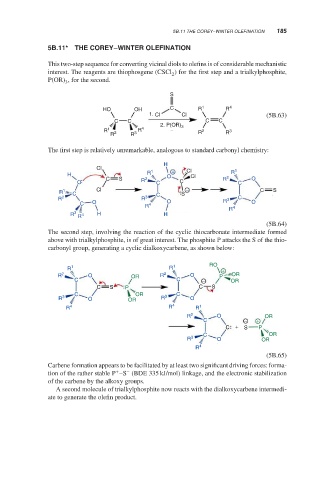
5B.11* THE COREY–WINTER OLEFINATION
This two-step sequence for converting vicinal diols to olefins is of considerable mechanistic
interest. The reagents are thiophosgene (CSCl ) for the first step and a trialkylphosphite,
2
P(OR) , for the second.
3
S
HO OH C R 1 R 4
1. Cl Cl (5B.63)
C C C C
2. P(OR) 3
R 1 R 4 R 2 R 3
R 2 R 3
The first step is relatively unremarkable, analogous to standard carbonyl chemistry:
H
Cl + Cl 1
H R 1 O Cl R
C S R 2 R 2 O
O C C C
R 1 C Cl C S − C S
R 2 R 3 R 3 C
C O 4 O O
R 4
R
R 3 R 4 H H
(5B.64)
The second step, involving the reaction of the cyclic thiocarbonate intermediate formed
above with trialkylphosphite, is of great interest. The phosphite P attacks the S of the thio-
carbonyl group, generating a cyclic dialkoxycarbene, as shown below:
R 1 R 1 RO +
R 2 O OR R 2 O P OR
C C − OR
C S P C S
C OR C
R 3 O OR R 3 O
R 4 R 4 R 1
R 2 O OR
C − +
C + S P
C OR
R 3 O OR
R 4
(5B.65)
Carbene formation appears to be facilitated by at least two significant driving forces: forma-
+
–
tion of the rather stable P –S (BDE 335 kJ/mol) linkage, and the electronic stabilization
of the carbene by the alkoxy groups.
A second molecule of trialkylphosphite now reacts with the dialkoxycarbene intermedi-
ate to generate the olefin product.