Page 201 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 201
5B.10 PHOSPHAZENES 181
REVIEW PROBLEM 5B.16
Suggest a mechanism for the general phosphazene synthesis mentioned above
(5B.49).
At present, phosphazenes are probably best known as neutral organic superbases, an
application pioneered by Reinhard Schwesinger in the 1980s. They are a big step up from
classic amine, amidine, and guanidine bases in terms of basicity. Two commonly used phos-
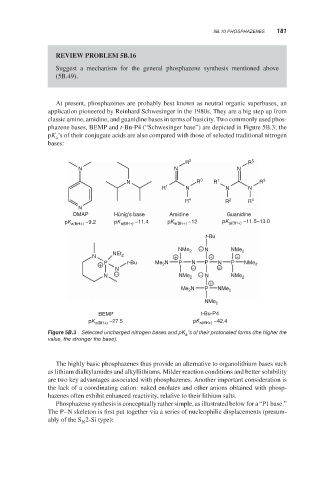
phazene bases, BEMP and t-Bu-P4 (“Schwesinger base”) are depicted in Figure 5B.3; the
pK ’s of their conjugate acids are also compared with those of selected traditional nitrogen
a
bases:
R 2 R 5
N N N
N R 3 R 1 R 3
R 1 N N N
R 4 R 2 R 4
N
DMAP Hünig’s base Amidine Guanidine
pK a(BH+) ~9.2 pK a(BH+) ~11.4 pK a(BH+) ~12 pK a(BH+) ~11.5–13.0
t-Bu
−
NMe 2 N NMe 2
N NEt 2 + + +
+ P t-Bu Me 2 N P N P N P NMe 2
N − −
N − NMe 2 − N NMe 2
+
Me 2 N P NMe 2
NMe 2
BEMP t-Bu-P4
pK a(BH+) ~27.5 pK a(BH+) ~42.4
Selected uncharged nitrogen bases and pK ’s of their protonated forms (the higher the
a
Figure 5B.3
value, the stronger the base).
The highly basic phosphazenes thus provide an alternative to organolithium bases such
as lithium dialkylamides and alkyllithiums. Milder reaction conditions and better solubility
are two key advantages associated with phosphazenes. Another important consideration is
the lack of a coordinating cation: naked enolates and other anions obtained with phosp-
hazenes often exhibit enhanced reactivity, relative to their lithium salts.
Phosphazene synthesis is conceptually rather simple, as illustrated below for a “P1 base.”
The P–N skeleton is first put together via a series of nucleophilic displacements (presum-
ably of the S 2-Si type):
N