Page 213 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 213
5B.15 SbF AND SUPERACIDS
5 193
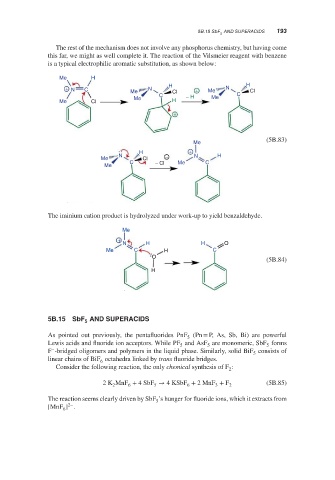
The rest of the mechanism does not involve any phosphorus chemistry, but having come
this far, we might as well complete it. The reaction of the Vilsmeier reagent with benzene
is a typical electrophilic aromatic substitution, as shown below:
Me H
H H
+ N C Me N Cl + Me N Cl
Me C − H Me C
Me Cl H
+
(5B.83)
Me
H +
N − N H
Me Cl
C − Cl Me C
Me
The iminium cation product is hydrolyzed under work-up to yield benzaldehyde.
Me
+
N H H O
Me C H C
O
(5B.84)
H
5B.15 SbF AND SUPERACIDS
5
As pointed out previously, the pentafluorides PnF (Pn = P, As, Sb, Bi) are powerful
5
Lewis acids and fluoride ion acceptors. While PF and AsF are monomeric, SbF forms
5
5
5
–
F -bridged oligomers and polymers in the liquid phase. Similarly, solid BiF consists of
5
linear chains of BiF octahedra linked by trans fluoride bridges.
6
Consider the following reaction, the only chemical synthesis of F :
2
2K MnF + 4 SbF → 4 KSbF + 2MnF + F (5B.85)
2 6 5 6 3 2
The reaction seems clearly driven by SbF ’s hunger for fluoride ions, which it extracts from
5
2–
[MnF ] .
6