Page 216 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 216
THE HEAVIER PNICTOGENS
196
• As mentioned, the inert pair effect is important for bismuth, which means that
pentavalent bismuth compounds are fairly strong oxidizing agents.
• Pn–C bond strengths also decrease as:
N > P > As > Sb > Bi
For example, for Ph Pn, the average Pn–C BDEs in kJ/mol are
3
Ph N ∶ 374 ± 4
3
Ph P ∶ 321 ± 21
3
Ph Bi ∶ 194 ± 11
3
Thus, many organobismuth compounds are stable enough for easy handling, but
exhibit useful group transfer reactivity on warming or other forms of activation.
• Finally, trivalent bismuth salts are attractive as Lewis acid catalysts in a number of
reactions.
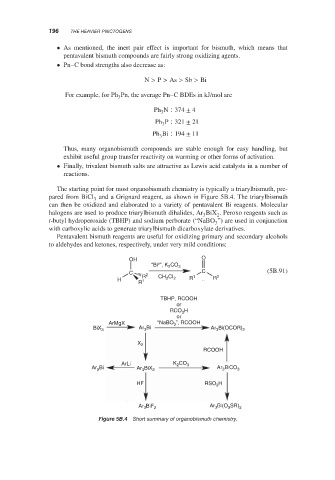
The starting point for most organobismuth chemistry is typically a triarylbismuth, pre-
pared from BiCl and a Grignard reagent, as shown in Figure 5B.4. The triarylbismuth
3
can then be oxidized and elaborated to a variety of pentavalent Bi reagents. Molecular
halogens are used to produce triarylbismuth dihalides, Ar BiX . Peroxo reagents such as
3 2
t-butyl hydroperoxide (TBHP) and sodium perborate (“NaBO ”) are used in conjunction
3
with carboxylic acids to generate triarylbismuth dicarboxylate derivatives.
Pentavalent bismuth reagents are useful for oxidizing primary and secondary alcohols
to aldehydes and ketones, respectively, under very mild conditions:
OH O
v
“Bi ”, K CO 3
2
C C (5B.91)
R 2 CH 2 CI 2 1 2
H 1 R R
R
TBHP, RCOOH
or
H
RCO 3
or
ArMgX “NaBO 3 ”, RCOOH
BiX 3 Ar Bi Ar Bi(OCOR) 2
3
3
X 2
RCOOH
ArLi K CO 3
2
Ar Bi Ar BiX 2 Ar BiCO 3
3
3
5
HF RSO 3 H
Ar BiF 2 Ar Bi(O SR) 2
3
3
3
Short summary of organobismuth chemistry.
Figure 5B.4