Page 195 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 195
5B.8 THE ARBUZOV REACTION 175
Hypophosphorous acid is an important reducing agent, both in chemistry labs and in
industry. Its best known use in organic chemistry is in the reduction of arenediazonium
+
cations (ArN ) to arenes (ArH). In industry, its chief application is “electroless” plating
2
where a metal such as nickel or copper is deposited on a surface by chemical reduction, as
opposed to passing an electric current (hence the name).
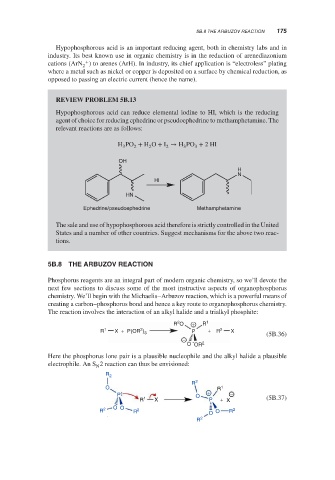
REVIEW PROBLEM 5B.13
Hypophosphorous acid can reduce elemental iodine to HI, which is the reducing
agent of choice for reducing ephedrine or pseudoephedrine to methamphetamine. The
relevant reactions are as follows:
H PO + H O + I → H PO + 2HI
2
3
2
2
3
3
OH
H
N
Hl
HN
Ephedrine/pseudoephedrine Methamphetamine
The sale and use of hypophosphorous acid therefore is strictly controlled in the United
States and a number of other countries. Suggest mechanisms for the above two reac-
tions.
5B.8 THE ARBUZOV REACTION
Phosphorus reagents are an integral part of modern organic chemistry, so we’ll devote the
next few sections to discuss some of the most instructive aspects of organophosphorus
chemistry. We’ll begin with the Michaelis–Arbuzov reaction, which is a powerful means of
creating a carbon–phosphorus bond and hence a key route to organophosphorus chemistry.
The reaction involves the interaction of an alkyl halide and a trialkyl phosphite:
2
R O + R 1
2
R 1 X + P(OR ) 3 P + R 2 X (5B.36)
−
O OR 2
Here the phosphorus lone pair is a plausible nucleophile and the alkyl halide a plausible
electrophile. An S 2 reaction can thus be envisioned:
N
R 2
R 2
O R 1
P O + −
R 1 X P + X (5B.37)
O O
R 2 R 2 O O R 2
R 2