Page 192 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 192
THE HEAVIER PNICTOGENS
172
H
H
O H H P H
H P +
P P
O (5B.28)
H 2
P P HO OH
P
HO
P
− OH
HO
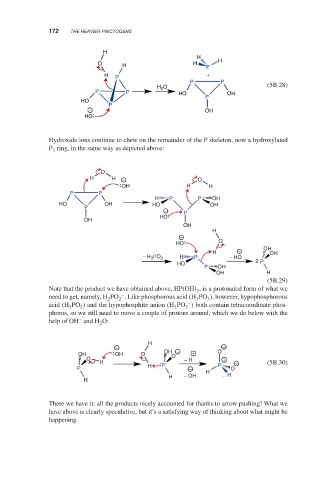
Hydroxide ions continue to chew on the remainder of the P skeleton, now a hydroxylated
P ring, in the same way as depicted above:
3
O
H H − O
OH H H
P P
H P P OH
HO OH HO OH
P
− P
HO
OH
OH
H
−
HO O
H − OH OH
− H PO 2 H P − HO
3
HO 2 P
P OH
OH H
(5B.29)
Note that the product we have obtained above, HP(OH) , is a protonated form of what we
2
–
need to get, namely, H PO . Like phosphorous acid (H PO ), however, hypophosphorous
2 2 3 3
–
acid (H PO ) and the hypophosphite anion (H PO ) both contain tetracoordinate phos-
3 2 2 2
phorus, so we still need to move a couple of protons around, which we do below with the
–
help of OH and H O:
2
H
− −
OH OH O OH − + O
O
O − H +
H P P − (5B.30)
P H − O
H
H − OH H
H
There we have it: all the products nicely accounted for thanks to arrow pushing! What we
have above is clearly speculative, but it’s a satisfying way of thinking about what might be
happening.