Page 194 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 194
THE HEAVIER PNICTOGENS
174
So we now have a pentavalent P with three hydrogens and two O ligands. A second hydride
transfer seems necessary to kick out one of the oxygens:
H
H −
P O − H O −
H + O O P H O + O
O P OH H P OH P P OH
H H O + H
H H
H H OH
H
(5B.34)
Deprotonation of the PH group (note that we have reoriented the molecule a bit below) and
4
simultaneous departure of phosphate—effectively a reductive elimination—now accounts
for the observed products. Below we have shown an intramolecular path for the process,
but an external base could also have accomplished the deprotonation:
H H − OH
H O OH + O OH
P P OH P P +
H + H (5B.35)
H O H OH
−
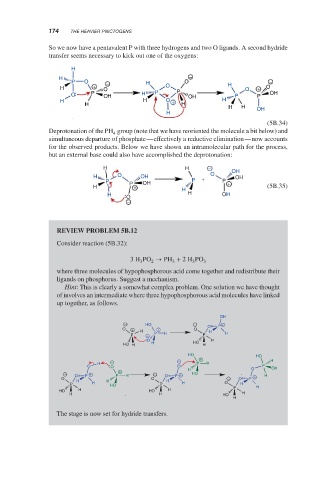
REVIEW PROBLEM 5B.12
Consider reaction (5B.32):
3H PO → PH + 2H PO 3
3
3
3
2
where three molecules of hypophosphorous acid come together and redistribute their
ligands on phosphorus. Suggest a mechanism.
Hint: This is clearly a somewhat complex problem. One solution we have thought
of involves an intermediate where three hypophosphorous acid molecules have linked
up together, as follows.
OH
− HO − O P +
O + H + O H
P − P H P H
O HO H
HO H H H
HO HO
− − + H
H P H
O O O O P OH
+ H
− O P + P H − O P + HO H
O O − O P +
H H H O
P H HO P H H H
HO H HO H P
H H HO H
H
The stage is now set for hydride transfers.