Page 187 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 187
5B.4 ARSENIC-BASED DNA 167
Although As-DNA and arsenic-based life do not seem grounded in reality, the episode
proved to be a catalyst for important discoveries on arsenic-tolerant organisms. One impor-
tant question that has been addressed is: how does GFAJ-1 tolerate such high concentrations
of arsenate as are found in Mono Lake and how does it discriminate between two such
similar species as phosphate and arsenate? A biochemical and crystallographic study on
phosphate-binding proteins (PBPs) from GFAJ-1 has shed significant light on this point
(Elias, M., et al. Nature 2012, 491, 124–137). First, the study showed that the PBPs indeed
4
3
bind phosphate 10 –10 times more strongly than they do arsenate. Second, they found
that arsenate binding leads to distortions in the hydrogen-bond network in the binding site,
which is finely tuned for phosphate, especially with respect to the geometry of one short
hydrogen bond. As far as phosphate/arsenate discrimination is concerned, GFAJ-1 does so
very well.
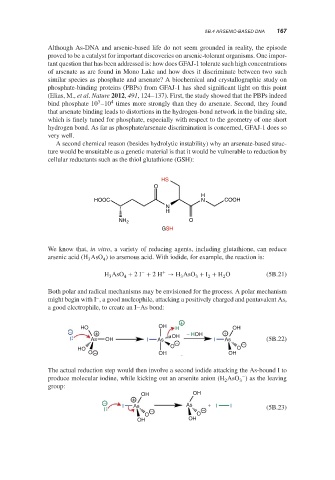
A second chemical reason (besides hydrolytic instability) why an arsenate-based struc-
ture would be unsuitable as a genetic material is that it would be vulnerable to reduction by
cellular reductants such as the thiol glutathione (GSH):
HS
O
H
HOOC N COOH
N
H
NH 2 O
GSH
We know that, in vitro, a variety of reducing agents, including glutathione, can reduce
arsenic acid (H AsO ) to arsenous acid. With iodide, for example, the reaction is:
4
3
− +
H AsO + 2I + 2H → H AsO + I + H O (5B.21)
3 4 3 3 2 2
Both polar and radical mechanisms may be envisioned for the process. A polar mechanism
–
might begin with I , a good nucleophile, attacking a positively charged and pentavalent As,
a good electrophile, to create an I–As bond:
+
HO OH H OH
− + − HOH +
I As OH I As OH I As (5B.22)
−
O −
HO O
O − OH OH
The actual reduction step would then involve a second iodide attacking the As-bound I to
–
produce molecular iodine, while kicking out an arsenite anion (H AsO ) as the leaving
2 3
group:
OH OH
+
− As + I I
I I As − − (5B.23)
O O
OH OH