Page 182 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 182
THE HEAVIER PNICTOGENS
162
+
–
As elsewhere in phosphorus chemistry, the great stability of the P –O unit provides the
driving force for this rearrangement.
REVIEW PROBLEM 5B.5
Phosphorous acid is a rather interesting molecule where the phosphorus oxidation
state (based on the formula H PO ), coordination number, and valence are all dif-
3
3
ferent, +3, 4, and 5, respectively! Explain and comment. If necessary, recall the
definitions of the terms from Chapter 1.
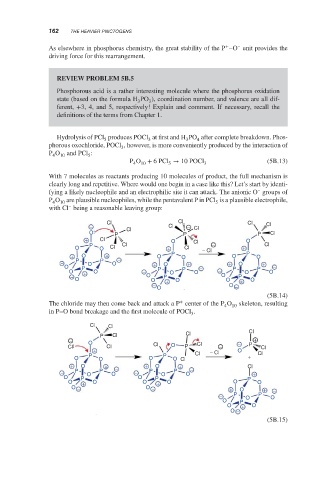
Hydrolysis of PCl produces POCl at first and H PO after complete breakdown. Phos-
5
4
3
3
phorous oxochloride, POCl , however, is more conveniently produced by the interaction of
3
P O 10 and PCl :
5
4
P O + 6PCl → 10 POCl 3 (5B.13)
10
4
5
With 7 molecules as reactants producing 10 molecules of product, the full mechanism is
clearly long and repetitive. Where would one begin in a case like this? Let’s start by identi-
–
fying a likely nucleophile and an electrophilic site it can attack. The anionic O groups of
P O are plausible nucleophiles, while the pentavalent P in PCl is a plausible electrophile,
4 10 5
–
with Cl being a reasonable leaving group:
Cl Cl Cl Cl
− Cl − Cl
O P Cl P P Cl
+ Cl O Cl O
P Cl − Cl
O O Cl + Cl − Cl +
+ O + O P O O P O
− P O P O − + O + + O +
O P P − P P −
O + O − O P O − O O
− O P O P
O O + O O + O
− O −
O
(5B.14)
+
The chloride may then come back and attack a P center of the P O skeleton, resulting
4 10
in P–O bond breakage and the first molecule of POCl .
3
Cl Cl
P Cl Cl Cl
− O +
Cl Cl Cl O P Cl − − P Cl
+ − Cl O
P P Cl + Cl
O O O O Cl
+ O + − + O + Cl
− P O P O − P O P O − +
O P O P P
O + O O + O O O
O − O − + O + −
− P O P O
O P
O + O
O −
(5B.15)