Page 178 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 178
THE HEAVIER PNICTOGENS
158
−
O
+
P(OCH ) P OCH 3
3 3
H C OCH 3
3
Suggest a mechanism for the reaction.
REVIEW PROBLEM 5B.2
Suggest a mechanism and a hard and soft Lewis acids and bases (HSAB) rationale
for the following ligand exchange (metathesis) reaction:
PCl + AsF → PF + AsCl 3
3
3
3
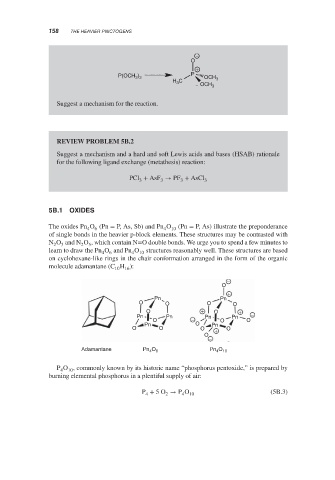
5B.1 OXIDES
The oxides Pn O (Pn = P, As, Sb) and Pn O 10 (Pn = P, As) illustrate the preponderance
4
4
6
of single bonds in the heavier p-block elements. These structures may be contrasted with
N O and N O , which contain N=O double bonds. We urge you to spend a few minutes to
2
3
2
5
learn to draw the Pn O and Pn O 10 structures reasonably well. These structures are based
4
6
4
on cyclohexane-like rings in the chair conformation arranged in the form of the organic
molecule adamantane (C H ):
16
10
−
O
+
Pn Pn
O O O O
O + O +
Pn Pn Pn Pn −
O − O O
Pn O Pn
O O O + O
O
−
Adamantane Pn 4 O 6 Pn O 10
4
P O , commonly known by its historic name “phosphorus pentoxide,” is prepared by
10
4
burning elemental phosphorus in a plentiful supply of air:
P + 5O → P O 10 (5B.3)
4
4
2