Page 174 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 174
NITROGEN
154
Its biological source is the essential amino acid arginine, from which it is synthesized
by the heme-containing enzyme NO synthase. Unfortunately, the mechanism is rather
complex, involving a good deal of iron (heme) chemistry, so we are obliged to skip it in
this book. Among NO’s myriad signaling roles, perhaps the best known is in vasodilation
in mammalian systems. NO is sensed by the enzyme-soluble guanylate cyclase (sGC),
another heme protein, which has a histidine ligand on the iron. NO binding to the heme iron
results in a loosening of the iron–histidine bond; the unligated histidine plays a key role in
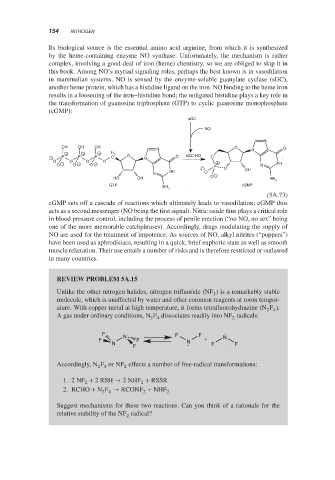
the transformation of guanosine triphosphate (GTP) to cyclic guanosine monophosphate
(cGMP):
sGC
NO
N
OH OH OH O
N N O
+ + + H 2
− P P P C O N O sGC-NO O
O O O O +
O − O − O − N NH
− P O OH
NH O
N
HO OH O − NH
2
GTP cGMP
NH 2
(5A.73)
cGMP sets off a cascade of reactions which ultimately leads to vasodilation; cGMP thus
acts as a second messenger (NO being the first signal). Nitric oxide thus plays a critical role
in blood pressure control, including the process of penile erection (“no NO, no sex” being
one of the more memorable catchphrases). Accordingly, drugs modulating the supply of
NO are used for the treatment of impotence. As sources of NO, alkyl nitrites (“poppers”)
have been used as aphrodisiacs, resulting in a quick, brief euphoric state as well as smooth
muscle relaxation. Their use entails a number of risks and is therefore restricted or outlawed
in many countries.
REVIEW PROBLEM 5A.15
Unlike the other nitrogen halides, nitrogen trifluoride (NF ) is a remarkably stable
3
molecule, which is unaffected by water and other common reagents at room temper-
ature. With copper metal at high temperature, it forms tetrafluorohydrazine (N F ).
2 4
A gas under ordinary conditions, N F dissociates readily into NF radicals:
2 4 2
F N F F
F F N + N
N F F
F
Accordingly, N F or NF effects a number of free-radical transformations:
2
2 4
1. 2 NF + 2RSH → 2 NHF + RSSR
2 2
2. RCHO + N F → RCONF + NHF
2 4 2 2
Suggest mechanisms for these two reactions. Can you think of a rationale for the
relative stability of the NF radical?
2