Page 171 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 171
5A.11 NITRIC OXIDE AND NITROGEN DIOXIDE 151
used to deliver the nitrene directly to an organic target molecule. We will discuss the role
of higher-valent bromine reagents as nitrene delivery agents in the chapter on halogens
(Section 7.14).
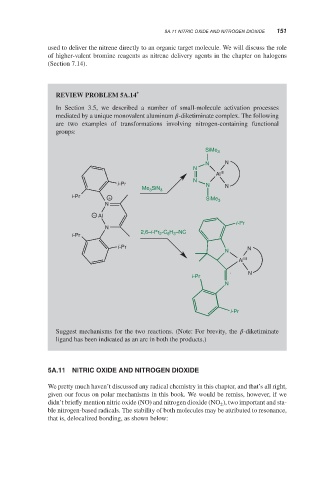
REVIEW PROBLEM 5A.14 *
In Section 3.5, we described a number of small-molecule activation processes
mediated by a unique monovalent aluminum -diketiminate complex. The following
are two examples of transformations involving nitrogen-containing functional
groups:
SiMe 3
N N
N
Al III
N
i-Pr N
Me SiN 3 N
3
i-Pr + SiMe 3
N
− Al
i-Pr
N
2,6–i-Pr 2 -C 6 H 3 –NC
i-Pr
i-Pr N
N
Al III
N
i-Pr
N
i-Pr
Suggest mechanisms for the two reactions. (Note: For brevity, the -diketiminate
ligand has been indicated as an arc in both the products.)
5A.11 NITRIC OXIDE AND NITROGEN DIOXIDE
We pretty much haven’t discussed any radical chemistry in this chapter, and that’s all right,
given our focus on polar mechanisms in this book. We would be remiss, however, if we
didn’t briefly mention nitric oxide (NO) and nitrogen dioxide (NO ), two important and sta-
2
ble nitrogen-based radicals. The stability of both molecules may be attributed to resonance,
that is, delocalized bonding, as shown below: