Page 170 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 170
NITROGEN
150
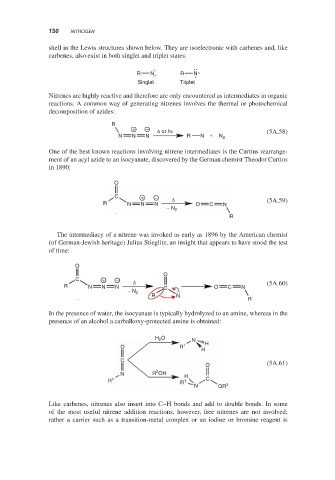
shell in the Lewis structures shown below. They are isoelectronic with carbenes and, like
carbenes, also exist in both singlet and triplet states:
R N R N
Singlet Triplet
Nitrenes are highly reactive and therefore are only encountered as intermediates in organic
reactions. A common way of generating nitrenes involves the thermal or photochemical
decomposition of azides:
R
+ − Δ or hν (5A.58)
N N N R N + N 2
One of the best known reactions involving nitrene intermediates is the Curtius rearrange-
ment of an acyl azide to an isocyanate, discovered by the German chemist Theodor Curtius
in 1890:
O
C + − Δ
R N N N O C N (5A.59)
− N 2
R
The intermediacy of a nitrene was invoked as early as 1896 by the American chemist
(of German-Jewish heritage) Julius Stieglitz, an insight that appears to have stood the test
of time:
O
O
C + − Δ
R N N N C O C N (5A.60)
− N 2
R N
R
In the presence of water, the isocyanate is typically hydrolyzed to an amine, whereas in the
presence of an alcohol a carbalkoxy-protected amine is obtained:
H O N
2
O R 1 H
H
C
O (5A.61)
2
N R OH H
R 1 R 1 C
N OR 2
Like carbenes, nitrenes also insert into C–H bonds and add to double bonds. In some
of the most useful nitrene addition reactions, however, free nitrenes are not involved;
rather a carrier such as a transition-metal complex or an iodine or bromine reagent is