Page 165 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 165
5A.8 IMINES AND RELATED FUNCTIONAL GROUPS 145
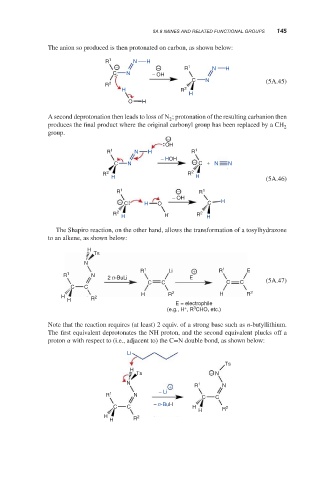
The anion so produced is then protonated on carbon, as shown below:
R 1 N H
− − R 1 N H
C N − OH
C N (5A.45)
R 2
H R 2
H
O H
A second deprotonation then leads to loss of N ; protonation of the resulting carbanion then
2
produces the final product where the original carbonyl group has been replaced by a CH
2
group.
−
OH
R 1 N H R 1
− HOH
C N − C + N N
R 2 R 2
H H (5A.46)
R 1 − R 1
− OH
− C H O C H
R 2 R 2
H H H
The Shapiro reaction, on the other hand, allows the transformation of a tosylhydrazone
to an alkene, as shown below:
H
Ts
N
R 1 Li + R 1 E
R 1 N
2 n-BuLi E
C C C C (5A.47)
C C
H R 2 H R 2
H 2
H R
E = electrophile
+
3
(e.g., H , R CHO, etc.)
Note that the reaction requires (at least) 2 equiv. of a strong base such as n-butyllithium.
The first equivalent deprotonates the NH proton, and the second equivalent plucks off a
proton with respect to (i.e., adjacent to) the C=N double bond, as shown below:
Li
Ts
H
Ts − N
N 1
+ R N
− Li
R 1 N
C C
− n-BuH
C C H 2
H R
H 2
H R