Page 162 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 162
NITROGEN
142
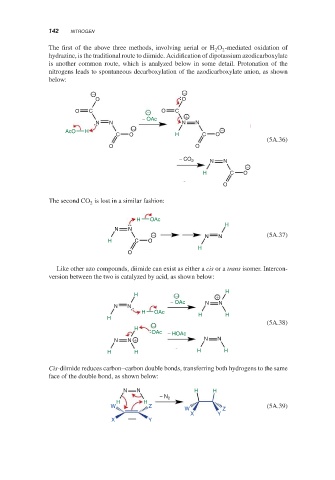
The first of the above three methods, involving aerial or H O -mediated oxidation of
2
2
hydrazine, is the traditional route to diimide. Acidification of dipotassium azodicarboxylate
is another common route, which is analyzed below in some detail. Protonation of the
nitrogens leads to spontaneous decarboxylation of the azodicarboxylate anion, as shown
below:
− −
O O
O C − O C
− OAc +
N N N N
− −
AcO H
C O H C O
(5A.36)
O O
− CO 2
N N
−
H C O
O
The second CO is lost in a similar fashion:
2
H OAc
H
N N
− N N (5A.37)
H C O
H
O
Like other azo compounds, diimide can exist as either a cis or a trans isomer. Intercon-
version between the two is catalyzed by acid, as shown below:
H
H − +
− OAc N N
N N
H OAc H H
H
− (5A.38)
H
OAc − HOAc
N N + N N
H H H H
Cis-diimide reduces carbon–carbon double bonds, transferring both hydrogens to the same
face of the double bond, as shown below:
N N H H
− N 2
H H
W Z (5A.39)
W Z
X Y
X Y