Page 163 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 163
5A.7 AZO COMPOUNDS AND DIAZENE 143
In this process, diimide reduction resembles catalytic hydrogenation (with metals such
as Pd, Pt, and Ni), which also follows the same stereochemical course, that is, delivers
H to a given face of the double bond. A disadvantage of diimide reductions is that
2
they tend to be significantly slower than catalytic hydrogenations, especially for tetra-
substituted and polarized double bonds. On the positive side, diimide reductions avoid
handling of the explosive H gas as well as removal and/or recovery of the transition-metal
2
catalysts.
Diimide reductions typically require an excess of the diimide-generating reagents,
because part of the diimide formed is lost to disproportionation:
2N H → N + N H (5A.40)
2 2 2 2 4
The mechanism is essentially the same as that involved in the reduction of a carbon–carbon
double bond:
N N H H
− N 2
H H N N
(5A.41)
N N
H H
H H
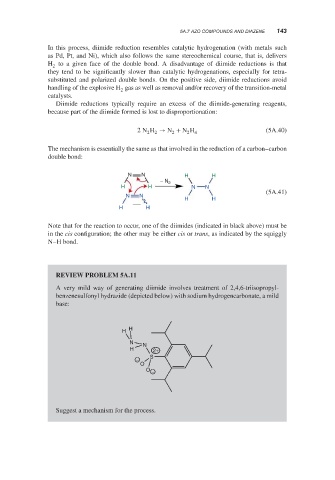
Note that for the reaction to occur, one of the diimides (indicated in black above) must be
in the cis configuration; the other may be either cis or trans, as indicated by the squiggly
N–H bond.
REVIEW PROBLEM 5A.11
A very mild way of generating diimide involves treatment of 2,4,6-triisopropyl-
benzenesulfonyl hydrazide (depicted below) with sodium hydrogencarbonate, a mild
base:
H H
N N
H 2+
− S
O
O −
Suggest a mechanism for the process.