Page 166 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 166
NITROGEN
146
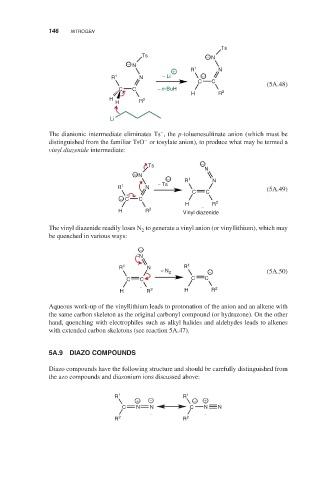
Ts
Ts − N
− N
+ R 1 N
R 1 N − Li −
C C
(5A.48)
C C − n-BuH 2
H R
H 2
H R
Li
–
The dianionic intermediate eliminates Ts ,the p-toluenesulfinate anion (which must be
−
distinguished from the familiar TsO or tosylate anion), to produce what may be termed a
vinyl diazenide intermediate:
−
Ts
N
− N
− R 1 N
R 1 N − Ts
C C (5A.49)
− C C
H R 2
H R 2 Vinyl diazenide
The vinyl diazenide readily loses N to generate a vinyl anion (or vinyllithium), which may
2
be quenched in various ways:
−
N
R 1 N R 1
− N 2 − (5A.50)
C C C C
H R 2 H R 2
Aqueous work-up of the vinyllithium leads to protonation of the anion and an alkene with
the same carbon skeleton as the original carbonyl compound (or hydrazone). On the other
hand, quenching with electrophiles such as alkyl halides and aldehydes leads to alkenes
with extended carbon skeletons (see reaction 5A.47).
5A.9 DIAZO COMPOUNDS
Diazo compounds have the following structure and should be carefully distinguished from
the azo compounds and diazonium ions discussed above:
R 1 R 1
+ − − +
C N N C N N
R 2 R 2