Page 168 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 168
NITROGEN
148
The loss of N is mediated by heat, light, or a transition-metal catalyst such as Ag O.
2
2
Typically, the unstable ketene is not isolated but is trapped by water (to yield a carboxylic
acid) or another molecule in the reaction medium.
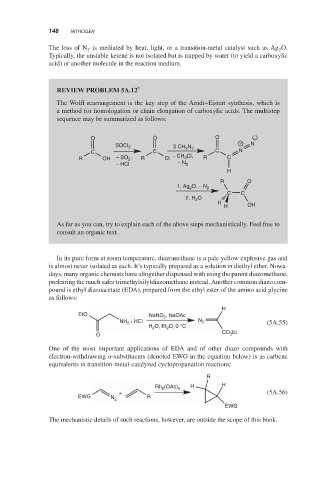
REVIEW PROBLEM 5A.12 *
The Wolff rearrangement is the key step of the Arndt–Eistert synthesis, which is
a method for homologation or chain elongation of carboxylic acids. The multistep
sequence may be summarized as follows:
O O O −
SOCI 2 2 CH 2 N 2 + N
C C C N
R OH − SO , R Cl − CH 3 Cl, R C
2
− HCI − N 2
H
R O O
1. Ag 2 O, − N 2
C C
2. H 2 O
H
H OH
As far as you can, try to explain each of the above steps mechanistically. Feel free to
consult an organic text.
In its pure form at room temperature, diazomethane is a pale yellow explosive gas and
is almost never isolated as such. It’s typically prepared as a solution in diethyl ether. Nowa-
days, many organic chemists have altogether dispensed with using the parent diazomethane,
preferring the much safer trimethylsilyldiazomethane instead. Another common diazo com-
pound is ethyl diazoacetate (EDA), prepared from the ethyl ester of the amino acid glycine
as follows:
H
EtO NaNO , NaOAc
2
• HCl N
NH 2 2 (5A.55)
O, Et O, 0 °C
H 2 2
Et
O CO 2
One of the most important applications of EDA and of other diazo compounds with
electron-withdrawing -substituents (denoted EWG in the equation below) is as carbene
equivalents in transition-metal-catalyzed cyclopropanation reactions:
R
H H
Rh 2 (OAc) 4
+ (5A.56)
EWG R
N 2
EWG
The mechanistic details of such reactions, however, are outside the scope of this book.