Page 172 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 172
NITROGEN
152
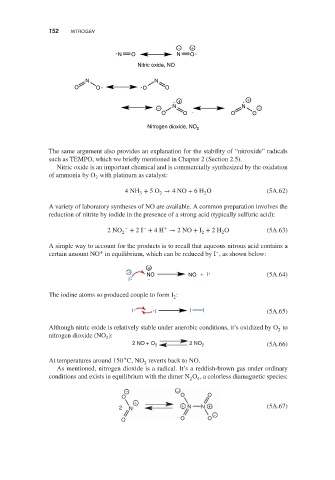
− +
N O N O
Nitric oxide, NO
N N
O O O O
+ +
− N N −
O O O O
Nitrogen dioxide, NO 2
The same argument also provides an explanation for the stability of “nitroxide” radicals
such as TEMPO, which we briefly mentioned in Chapter 2 (Section 2.5).
Nitric oxide is an important chemical and is commercially synthesized by the oxidation
of ammonia by O with platinum as catalyst:
2
4NH + 5O → 4NO + 6H O (5A.62)
3 2 2
A variety of laboratory syntheses of NO are available. A common preparation involves the
reduction of nitrite by iodide in the presence of a strong acid (typically sulfuric acid):
− − +
2NO + 2I + 4H → 2NO + I + 2H O (5A.63)
2 2 2
A simple way to account for the products is to recall that aqueous nitrous acid contains a
+ –
certain amount NO in equilibrium, which can be reduced by I , as shown below:
+
−
NO NO + I (5A.64)
I
The iodine atoms so produced couple to form I :
2
I I I I (5A.65)
Although nitric oxide is relatively stable under anerobic conditions, it’s oxidized by O to
2
nitrogen dioxide (NO ):
2
2 NO + O 2 2 NO 2 (5A.66)
∘
At temperatures around 150 C, NO reverts back to NO.
2
As mentioned, nitrogen dioxide is a radical. It’s a reddish-brown gas under ordinary
conditions and exists in equilibrium with the dimer N O , a colorless diamagnetic species:
2
4
− −
O O O
+
2 N + N N + (5A.67)
−
O O O