Page 167 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 167
5A.9 DIAZO COMPOUNDS 147
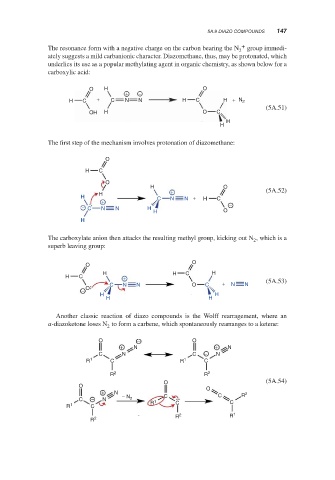
The resonance form with a negative charge on the carbon bearing the N + group immedi-
2
ately suggests a mild carbanionic character. Diazomethane, thus, may be protonated, which
underlies its use as a popular methylating agent in organic chemistry, as shown below for a
carboxylic acid:
O H O
+ −
H C + C N N H C H + N 2
(5A.51)
OH H O C
H
H
The first step of the mechanism involves protonation of diazomethane:
O
H C
O
H O
H + (5A.52)
H C N N + H C
+
− C N N H −
H O
H
The carboxylate anion then attacks the resulting methyl group, kicking out N , which is a
2
superb leaving group:
O
O
H H C H
H C +
C N N O C + N N (5A.53)
− O
H H
H H
Another classic reaction of diazo compounds is the Wolff rearragement, where an
-diazoketone loses N to form a carbene, which spontaneously rearranges to a ketene:
2
O − O
+ N + N
C N C − N
R 1 C R 1 C
R 2 R 2
O (5A.54)
O O
+ N C R 2
C − N − N 2 1 C C
R 1 C R C
R 2 R 1
R 2