Page 179 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 179
5B.1 OXIDES 159
P O 10 is best known as a powerful dehydrating agent. Not only does it react avidly with
4
water but it also extracts the elements of water from various substances. In Section 7.7, we’ll
go through a remarkable reaction in which P O 10 dehydrates perchloric acid (HClO ), one
4
4
of the strongest common acids, to Cl O . A typical acidic oxide, P O reacts with water,
2 7 4 10
producing phosphoric acid (H PO ):
3 4
P O + 6H O → 4H PO (5B.4)
4 10 2 3 4
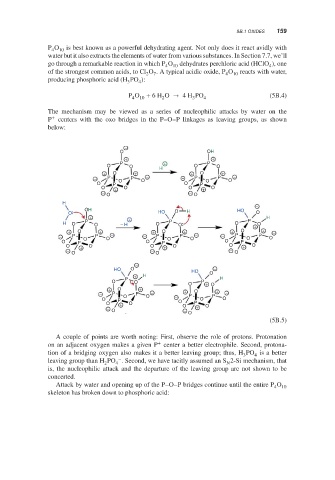
The mechanism may be viewed as a series of nucleophilic attacks by water on the
+
P centers with the oxo bridges in the P–O–P linkages as leaving groups, as shown
below:
−
O OH
+ +
P + P
O O O O
H
+ O + + O +
− P O P O − − P O P O −
O P O P
O + O O + O
− O − O
H
−
OH O H HO
O HO O
+ H
P + P P
H O O − H O O O + O
+ O + + O + + O + −
− P O P O − − P O P O − − P O P O
O P O P O P
O + O O + O O + O
− − − O
O O
−
HO O −
HO O
+ H
P H
O O O P + O
+ O + + O +
− P O P O − P P −
O P − O O
O + O O O P O
− O − O +
(5B.5)
A couple of points are worth noting: First, observe the role of protons. Protonation
+
on an adjacent oxygen makes a given P center a better electrophile. Second, protona-
tion of a bridging oxygen also makes it a better leaving group; thus, H PO is a better
3
4
–
leaving group than H PO . Second, we have tacitly assumed an S 2-Si mechanism, that
4
2
N
is, the nucleophilic attack and the departure of the leaving group are not shown to be
concerted.
Attack by water and opening up of the P–O–P bridges continue until the entire P O 10
4
skeleton has broken down to phosphoric acid: