Page 108 - Artificial Intelligence for Computational Modeling of the Heart
P. 108
78 Chapter 2 Implementation of a patient-specific cardiac model
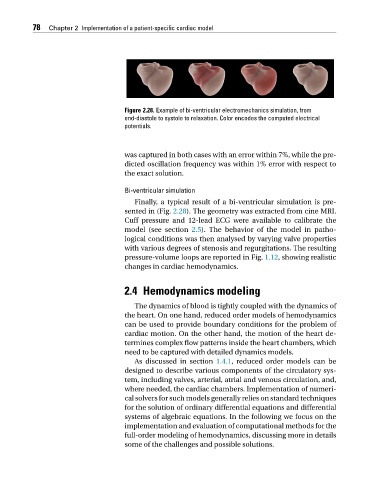
Figure 2.28. Example of bi-ventricular electromechanics simulation, from
end-diastole to systole to relaxation. Color encodes the computed electrical
potentials.
was captured in both cases with an error within 7%, while the pre-
dicted oscillation frequency was within 1% error with respect to
the exact solution.
Bi-ventricular simulation
Finally, a typical result of a bi-ventricular simulation is pre-
sented in (Fig. 2.28). The geometry was extracted from cine MRI.
Cuff pressure and 12-lead ECG were available to calibrate the
model (see section 2.5). The behavior of the model in patho-
logical conditions was then analysed by varying valve properties
with various degrees of stenosis and regurgitations. The resulting
pressure-volume loops are reported in Fig. 1.12, showing realistic
changes in cardiac hemodynamics.
2.4 Hemodynamics modeling
The dynamics of blood is tightly coupled with the dynamics of
the heart. On one hand, reduced order models of hemodynamics
can be used to provide boundary conditions for the problem of
cardiac motion. On the other hand, the motion of the heart de-
termines complex flow patterns inside the heart chambers, which
need to be captured with detailed dynamics models.
As discussed in section 1.4.1, reduced order models can be
designed to describe various components of the circulatory sys-
tem, including valves, arterial, atrial and venous circulation, and,
where needed, the cardiac chambers. Implementation of numeri-
cal solvers for such models generally relies on standard techniques
for the solution of ordinary differential equations and differential
systems of algebraic equations. In the following we focus on the
implementation and evaluation of computational methods for the
full-order modeling of hemodynamics, discussing more in details
some of the challenges and possible solutions.