Page 87 - Artificial Intelligence for Computational Modeling of the Heart
P. 87
Chapter 2 Implementation of a patient-specific cardiac model 57
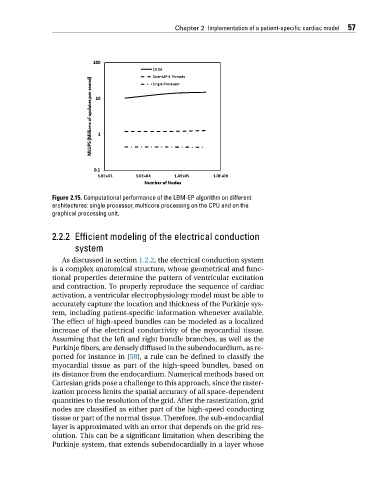
Figure 2.15. Computational performance of the LBM-EP algorithm on different
architectures: single processor, multicore processing on the CPU and on the
graphical processing unit.
2.2.2 Efficient modeling of the electrical conduction
system
As discussed in section 1.2.2, the electrical conduction system
is a complex anatomical structure, whose geometrical and func-
tional properties determine the pattern of ventricular excitation
and contraction. To properly reproduce the sequence of cardiac
activation, a ventricular electrophysiology model must be able to
accurately capture the location and thickness of the Purkinje sys-
tem, including patient-specific information whenever available.
The effect of high-speed bundles can be modeled as a localized
increase of the electrical conductivity of the myocardial tissue.
Assuming that the left and right bundle branches, as well as the
Purkinje fibers, are densely diffused in the subendocardium, as re-
ported for instance in [58], a rule can be defined to classify the
myocardial tissue as part of the high-speed bundles, based on
its distance from the endocardium. Numerical methods based on
Cartesian grids pose a challenge to this approach, since the raster-
ization process limits the spatial accuracy of all space-dependent
quantities to the resolution of the grid. After the rasterization, grid
nodes are classified as either part of the high-speed conducting
tissue or part of the normal tissue. Therefore, the sub-endocardial
layer is approximated with an error that depends on the grid res-
olution. This can be a significant limitation when describing the
Purkinje system, that extends subendocardially in a layer whose