Page 88 - Artificial Intelligence for Computational Modeling of the Heart
P. 88
58 Chapter 2 Implementation of a patient-specific cardiac model
thickness may be of the same order of magnitude of the grid spac-
ing (up to one third or one half of the ventricular thickness). Spe-
cialized methods can be designed, able to provide sub-grid spatial
accuracy, thus reproducing the same effect of high-speed con-
ducting tissue independently of the resolution of the Cartesian
grid.
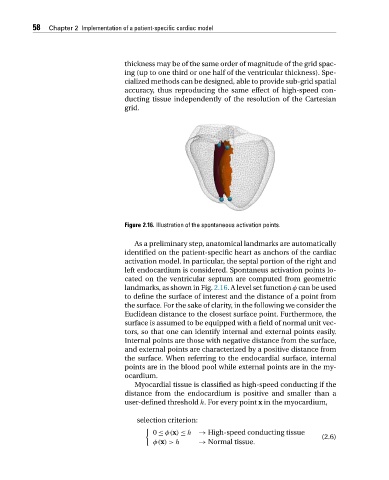
Figure 2.16. Illustration of the spontaneous activation points.
As a preliminary step, anatomical landmarks are automatically
identified on the patient-specific heart as anchors of the cardiac
activation model. In particular, the septal portion of the right and
left endocardium is considered. Spontaneus activation points lo-
cated on the ventricular septum are computed from geometric
landmarks, as shown in Fig. 2.16.Alevelsetfunction φ can be used
to define the surface of interest and the distance of a point from
the surface. For the sake of clarity, in the following we consider the
Euclidean distance to the closest surface point. Furthermore, the
surface is assumed to be equipped with a field of normal unit vec-
tors, so that one can identify internal and external points easily.
Internal points are those with negative distance from the surface,
and external points are characterized by a positive distance from
the surface. When referring to the endocardial surface, internal
points are in the blood pool while external points are in the my-
ocardium.
Myocardial tissue is classified as high-speed conducting if the
distance from the endocardium is positive and smaller than a
user-defined threshold h. For every point x in the myocardium,
selection criterion:
0 ≤ φ(x) ≤ h → High-speed conducting tissue
(2.6)
φ(x)>h → Normal tissue.