Page 62 - Basic Gas Chromatography
P. 62
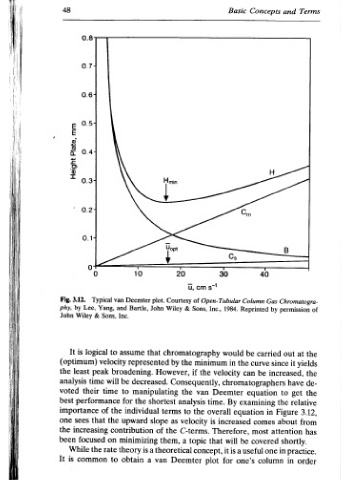
Terms Rate The Theory 49 to conditions. operating the and it evaluate run and chosen is solute A flow of variety a at isothermally sufficient allow to sure being rates, time after equilibration pressure for number plate The change. each evaluated is chromatogram each from then and 19 equation using the calculate to used (equation height plate values
and B Chromatogra- permission out since it increased, have get to the Figure about attention shortly. in in
Concepts 40 Gas Column by Reprinted carried curve be chromatographers equation examining in comes most covered useful one column
Basic g 1984. would be the in can By equation increased Therefore, be will a is one’s
30 Open- Tubular velocity Deemter overall it for
s71 Inc., minimum time. is that concept, plot
om of Sons, the Consequently, van the topic
u, & chromatography the if the analysis to velocity C-terms. Deemter
20 Courtesy a
& Wiley by as theoretical
£ - Uopt However, terms the them,
rz shortest
plot. John that represented decreased. manipulating slope of a van a
10 Deemter Bartle, assume broadening. the individual upward contribution minimizing is theory obtain
van and Inc. to velocity be will to time for the the on to
Typical Yang, Sons, & logical peak time performance of that the rate
0.8 0.77 ° + o a 0.14 5 Lee, Wiley their increasing focused common
wn
3.12. by is (optimum) least importance sees While
°
°
Wi ‘aze}q }YBie} It analysis is
48 Fig. phy, John the voted best one the been It