Page 343 - Battery Reference Book
P. 343
30/14 Primary batteries
140
130
120
110
100
>
.g 90
0
;
-
80
70
0
0
m 4 6C
C
:
n 5c
4c
3c
2c
1(
( I I I I I I I
3 -20 -10 0 10 20 30 40
Cell temperature ("C) Cell temperature ("C)
la) Carbon-zinc type [b) Carbon-zinc chloride type
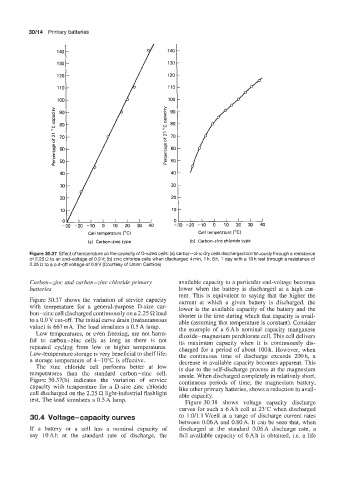
Figure 30.37 Effect of temperature on the capacity of D-sized cells: (a) carbon-zinc dry cells discharged continuously through a resistance
of 2.25 R to an end-voltage of 0.9V; (b) zinc chloride cells when discharged 4rnin, 1 h, 8 h, 1 day with a 16 h rest through a resistance of
2.25 S2 to a cut-off voltage of 0.9V (Courtesy of Union Carbide)
Carbon-zinc and carbon-zinc chloride primary available capacity to a particular end-voltage becomes
batteries lower when the battery is discharged at a high cur-
rent. This is equivalent to saying that the higher the
Figure 30.37 shows the variation of service capacity current at which a given battery is discharged, the
with temperature for a general-purpose D-size car- lower is the available capacity of the battery and the
bon-zinc cell discharged continuously on a 2.25 C2 load shorter is the time during which that capacity is avail-
to a 0.9 V cut-off. The initial curve drain (instantaneous able (assuming that temperature is constant). Consider
value) is 667 mA. The load simulates a 0.5 A lamp. the example of a 6Ah nominal capacity manganese
Low temperatures, or even freezing, are not harm- dioxide-magnesium perchlorate cell. This cell delivers
ful to carbon-zinc cells as long as there is not its maximum capacity when it is continuously dis-
repeated cycling from low or higher temperatures. charged for a period of about 100 h. However, when
Low-temperature storage is very beneficial to shelf life; the continuous time of discharge exceeds 200 h, a
a storage temperature of 4-10°C is effective. decrease in available capacity becomes apparent. This
The zinc chloride cell performs better at low is due to the self-discharge process at the magnesium
temperatures than the standard carbon-zinc cell. anode. When discharged completely in relatively short,
Figure 30.37(b) indicates the variation of service continuous periods of time, the magnesium battery,
capacity with temperature for a D-size zinc chloride like other primary batteries, shows a reduction in avail-
cell discharged on the 2.25 C? light-industrial flashlight able capacity.
test. The load simulates a 0.5 A lamp. Figure 30.38 shows voltage capacity discharge
curves for such a 6 Ah cell at 23°C when discharged
30.4 Voltage-capacity curves to 1.0/1.1 V/cell at a range of discharge current rates
between 0.06 A and 0.80 A. It can be seen that, when
If a battery or a cell has a nominal capacity of discharged at the standard 0.06A discharge rate, a
say lOAh at the standard rate of discharge, the full available capacity of 6 Ah is obtained, i.e. a life