Page 476 - Battery Reference Book
P. 476
Types of constant-current charging 47/9
of charge. Overcharge acceptance at this rate is great case of Nife Jungner sealed nickel-cadmium cells in
(more than 20 000 h). Sealed rectangular cells must be Table 47.2. Continued charging to full state of charge
in the dischagEd state before being recharged for 14 h must not be carried out with this high current, but the
at O.lCsA. Below 10°C the rate of charge must be normal charging currents shown in Table 47.2 should
reduced. be used. This requires several hours and thus charging
The 10h rate should not normally be exceeded to full charge with this method is not advantageous. In
unless overcharge is specifically to be prevented. Dur- urgent situations, when full capacity is not required, it
ing charging, the cell voltage rises from approximately can be useful, however.
1.30 V to a maximum of approximately 1.45 V at near
full charge. Finally, the voltage is stabilized at approx- 47.3.3 Controlled sapid constant-current
imately 1.43 V. The decreasing voltage at the end of
charging depends on a temperature rise in the cell. It charging
is caused by the reaction between oxygen and the neg- This is understood to imply a charge period of less than
ative electrode in the cell. This voltage characteristic, 1 h. (When the period is less than 15 min the charge is
with a gradual and relatively moderate voltage increase called ultrarapid; see below.) Controlled rapid charge
followed by stabilizing at a level slightly less than implies that there is a system that interrupts the charge
maximum. is typical for sealed nickel-cadmium cells. before the cells or battery reaches an overchaxge state
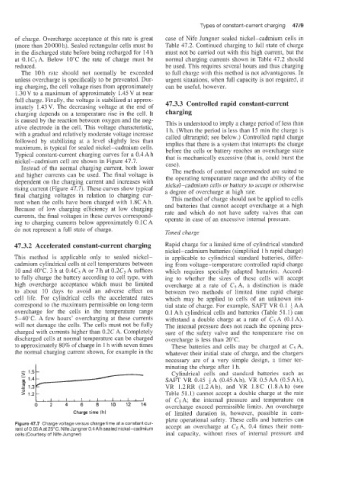
Typical constant-current charging curves for a 0.4 Ah that is mechanically excessive (that is, could burst the
nickel-cadmium cell are shown in Figure 47.7. case).
Instead of the normal charging current, both lower
The methods of control recommended are suited to
and higher currents can be used. The final voltage is the operating temperature range and the ability of the
dependent on the charging current and increases with nickel-cadmium cells or battery to accept or otherwise
rising current (Figure 47.7). These curves show typical a degree of overcharge at high rate.
final charging voltages in relation to charging cur- This method of charge should not be applied to cells
rent when the cells have been charged with 1.8CAh. and batteries that cannot accept overcharge at a high
Because of low charging efficiency at low charging rate and which do not have safety valves that can
currents, the final voltages in these curves correspond-
ing to charging currents below approximately 0.1C A operate in case of an excessive internal pressure.
do not represent a full state of charge. Timed charge
47.3.2 Accelerated constant-current charging Rapid charge for a limited time of cylindrical standard
nickel-cadmium batteries (simplified 1 h rapid charge)
This method is applicable only to sealed nickel- is applicable to cylindrical standard batteries, differ-
cadmium cylindrical cells at cell temperatures between ing from voltage-temperature controlled rapid charge
10 and 40°C. 3 h at 0.4c5 A or 7 h at 0.2C5 A suffices which requires specially adapted batteries. Accord-
to fully charge the battery according to cell type, with ing to whether the sizes of these cells will accept
high overcharge acceptance which must be limited overcharge at a rate of Cs A, a distinction is made
to about 10 days to avoid an adverse effect on between two methods of limited time rapid charge
cell life. For cylindrical cells the accelerated rates which may be applied to cells of an unknown ini-
correspond to the maximum permissible on long-term tial state of charge. For example, SAFT VR 0.1 f AA
overcharge for the cells in the temperature range 0.1 Ah cylindrical cells and batteries (Table 5 1.1) can
5540°C. A few hours’ overcharging at these currents withstand a double charge at a rate of C5 A (0.1 A).
will not damage the cells. The cells must not be fully The internal pressure does not reach the opening pres-
charged with currents higher than 0.2C A. Completely sure of the safety valve and the temperature rise on
discharged cells at normal temperature can be charged overcharge is less than 20°C.
to approximately 80% of charge in 1 h with seven times These batteries and cells may be charged at C5 A,
the normal charging current shown, for example in the whatever their initial state of charge, and the chargers
necessary are of a very simple design, a timer ter-
minating the charge after 1 h.
Cylindrical cells and standard batteries such as
SAFT VR 0.45 1 A (0.45 Ah), VR 0.5 AA (0.5 Ah),
VR 1.2RR (1.2Ah), and VR 1.8C (1.8Ah) (see
Table 51.1) cannot accept a double charge at the rate
of CsA; the internal pressure and temperature on
0 2 4 6 8 101214 overcharge exceed permissible limits. An overcharge
Charge time (h) of limited duration is, however, possible in com-
plete operational safety. These cells and batteries can
Figure 47.7 Charge voltage versus charge time at a constant cur- accept an overcharge at Cg A, 0.4 times their nom-
rent of 0.05A at 25°C. Nife Jungner0.4A h sealed nickel-cadmium
cells (Courtesy of Nife Jungner) inal capacity, without rises of internal pressure and