Page 69 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 69
3.1 Introduction 45
synthesis. At that time, most chemists were used to conventional oxidants and it
was neither appealing nor often possible for them to cultivate rare organisms such
as, for instance, Acinetobacter calcoaceticus and to isolate specific enzymes from
microbial strains. Nevertheless, mild stereo- and regioselective oxidants would
have complemented the classical chemistry oxidation toolbox and would have been
highly beneficial. However, there was another significant drawback in the synthetic
exploitation of many redox enzymes, namely the necessity of using expensive
+
cofactors such as NAD(P)H or the oxidized NAD(P) . These cofactors needed to be
applied either in stoichiometric amounts unless a cofactor recycling method had
been implemented.
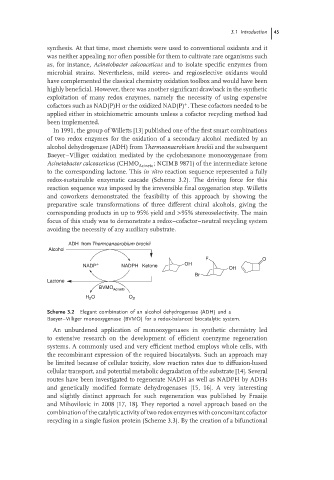
In 1991, the group of Willetts [13] published one of the first smart combinations
of two redox enzymes for the oxidation of a secondary alcohol mediated by an
alcohol dehydrogenase (ADH) from Thermoanaerobium brockii and the subsequent
Baeyer–Villiger oxidation mediated by the cyclohexanone monooxygenase from
Acinetobacter calcoaceticus (CHMO Acineto ; NCIMB 9871) of the intermediate ketone
to the corresponding lactone. This in vitro reaction sequence represented a fully
redox-sustainable enzymatic cascade (Scheme 3.2). The driving force for this
reaction sequence was imposed by the irreversible final oxygenation step. Willetts
and coworkers demonstrated the feasibility of this approach by showing the
preparative scale transformations of three different chiral alcohols, giving the
corresponding products in up to 95% yield and >95% stereoselectivity. The main
focus of this study was to demonstrate a redox–cofactor–neutral recycling system
avoiding the necessity of any auxiliary substrate.
ADH from Thermoanaerobium brockii
Alcohol
F O
+ OH
NADP NADPH Ketone
OH
Br
Lactone
BVMO Acineto
H O O 2
2
Scheme 3.2 Elegant combination of an alcohol dehydrogenase (ADH) and a
Baeyer–Villiger monooxygenase (BVMO) for a redox-balanced biocatalytic system.
An unburdened application of monooxygenases in synthetic chemistry led
to extensive research on the development of efficient coenzyme regeneration
systems. A commonly used and very efficient method employs whole cells, with
the recombinant expression of the required biocatalysts. Such an approach may
be limited because of cellular toxicity, slow reaction rates due to diffusion-based
cellular transport, and potential metabolic degradation of the substrate [14]. Several
routes have been investigated to regenerate NADH as well as NADPH by ADHs
and genetically modified formate dehydrogenases [15, 16]. A very interesting
and slightly distinct approach for such regeneration was published by Fraaije
and Mihovilovic in 2008 [17, 18]. They reported a novel approach based on the
combination of the catalytic activity of two redox enzymes with concomitant cofactor
recycling in a single fusion protein (Scheme 3.3). By the creation of a bifunctional