Page 71 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 71
3.1 Introduction 47
O 2
Gaseous phase
O
O OH
Organic phase
Aqueous phase O
O
E. coli cell
O NADH
H 2 FAD
H +
StyA StyB LSADH
+
O 2 FADH 2 NAD
O 2
OH
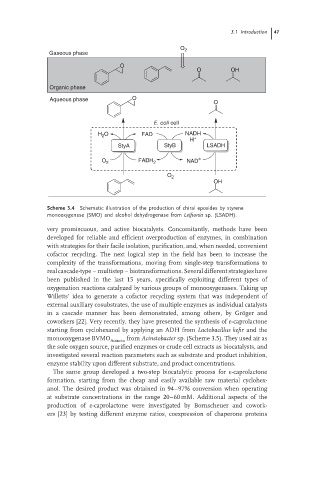
Scheme 3.4 Schematic illustration of the production of chiral epoxides by styrene
monooxygenase (SMO) and alcohol dehydrogenase from Leifsonia sp. (LSADH).
very promiscuous, and active biocatalysts. Concomitantly, methods have been
developed for reliable and efficient overproduction of enzymes, in combination
with strategies for their facile isolation, purification, and, when needed, convenient
cofactor recycling. The next logical step in the field has been to increase the
complexity of the transformations, moving from single-step transformations to
real cascade-type – multistep – biotransformations. Several different strategies have
been published in the last 15 years, specifically exploiting different types of
oxygenation reactions catalyzed by various groups of monooxygenases. Taking up
Willetts’ idea to generate a cofactor recycling system that was independent of
external auxiliary cosubstrates, the use of multiple enzymes as individual catalysts
in a cascade manner has been demonstrated, among others, by Gr¨ oger and
coworkers [22]. Very recently, they have presented the synthesis of ε-caprolactone
starting from cyclohexanol by applying an ADH from Lactobacillus kefir and the
monooxygenase BVMO Acineto from Acinetobacter sp. (Scheme 3.5). They used air as
the sole oxygen source, purified enzymes or crude cell extracts as biocatalysts, and
investigated several reaction parameters such as substrate and product inhibition,
enzyme stability upon different substrate, and product concentrations.
The same group developed a two-step biocatalytic process for ε-caprolactone
formation, starting from the cheap and easily available raw material cyclohex-
anol. The desired product was obtained in 94–97% conversion when operating
at substrate concentrations in the range 20–60 mM. Additional aspects of the
production of ε-caprolactone were investigated by Bornscheuer and cowork-
ers [23] by testing different enzyme ratios, coexpression of chaperone proteins