Page 73 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 73
3.1 Introduction 49
transformations, which is due to the fact that the employed styrol monooxygenase
is not capable of transforming carbocylic acid-containing styrol derivatives. Con-
sequently, the biocatalyzed cascade transformation could be performed only in a
sequential manner, and the products were isolated in quantitative yields and an
enantiomeric excess greater than 95%.
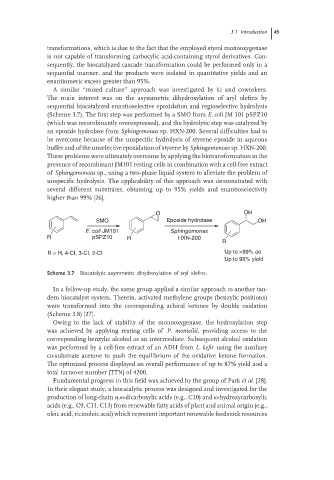
A similar ‘‘mixed culture’’ approach was investigated by Li and coworkers.
The main interest was on the asymmetric dihydroxylation of aryl olefins by
sequential biocatalyzed enantioselective epoxidation and regioselective hydrolysis
(Scheme 3.7). The first step was performed by a SMO from E. coli JM 101 pSPZ10
(which was recombinantly overexpressed), and the hydrolytic step was catalyzed by
an epoxide hydrolase from Sphingomonas sp. HXN-200. Several difficulties had to
be overcome because of the unspecific hydrolysis of styrene epoxide in aqueous
buffer and of the unselective epoxidation of styrene by Sphingomonoas sp. HXN-200.
These problems were ultimately overcome by applying the biotransformation in the
presence of recombinant JM101 resting cells in combination with a cell-free extract
of Sphingomonoas sp., using a two-phase liquid system to alleviate the problem of
unspecific hydrolysis. The applicability of this approach was demonstrated with
several different substrates, obtaining up to 95% yields and enantioselectivity
higher than 99% [26].
O OH
SMO Epoxide hydrolase OH
E. coli JM101 Sphingomonas
R pSPZ10 R HXN-200
R
R = H, 4-CI, 3-CI, 2-CI Up to >99% ee
Up to 95% yield
Scheme 3.7 Biocatalytic asymmetric dihydroxylation of aryl olefins.
In a follow-up study, the same group applied a similar approach to another tan-
dem biocatalyst system. Therein, activated methylene groups (benzylic positions)
were transformed into the corresponding achiral ketones by double oxidation
(Scheme 3.8) [27].
Owing to the lack of stability of the monooxygenase, the hydroxylation step
was achieved by applying resting cells of P. monteilii, providing access to the
corresponding benzylic alcohol as an intermediate. Subsequent alcohol oxidation
was performed by a cell-free extract of an ADH from L. kefir using the auxiliary
co-substrate acetone to push the equilibrium of the oxidative ketone formation.
The optimized process displayed an overall performance of up to 87% yield and a
total turnover number (TTN) of 4200.
Fundamental progress in this field was achieved by the group of Park et al. [28].
In their elegant study, a biocatalytic process was designed and investigated for the
production of long-chain α,ω-dicarboxylic acids (e.g., C10) and ω-hydroxycarboxylic
acids (e.g., C9, C11, C13) from renewable fatty acids of plant and animal origin (e.g.,
oleic acid, ricinoleic acid) which represent important renewable feedstock resources