Page 77 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 77
3.1 Introduction 53
Initially, they fully characterized each individual step of this cascade, investigating
the mechanistic and kinetic aspects. Simply by modifying the reaction parameters
and varying the media composition (different substrate and intermediate concentra-
tions) without changing the gene expression levels, they could control the reaction
to stop at the aldehyde or carboxylic acid level. By employing a two-liquid phase
system and supplying distinct amounts of pseudocumene or 3,4-dimethylbenzyl
alcohol, the oxidation process yielded the corresponding aldehydes exclusively. A
productivity of 31 g l −1 d −1 of 3,4-dimethylbenzaldehyde was obtained [31], ulti-
mately demonstrating the industrial applicability of this process and underscoring
the potential of monooxygenases for biotechnology [32].
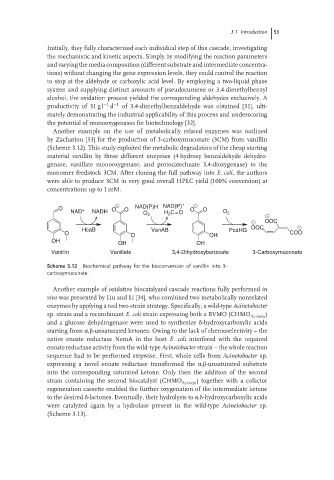
Another example on the use of metabolically related enzymes was outlined
by Zachariou [33] for the production of 3-carboxymuconate (3CM) from vanillin
(Scheme 3.12). This study exploited the metabolic degradation of the cheap starting
material vanillin by three different enzymes (4-hydroxy benzaldehyde dehydro-
genase, vanillate monooxygenase, and protocatechuate 3,4-dioxygenase) to the
monomer feedstock 3CM. After cloning the full pathway into E. coli, the authors
were able to produce 3CM in very good overall HPLC yield (100% conversion) at
concentrations up to 1 mM.
NAD(P)H NAD(P) +
O + O O O O
NAD NADH H C=O O 2
O 2 2
OOC
HcaB VanAB PcaHG OOC
O O OH COO
OH
OH OH
Vanillin Vanillate 3,4-Dihydroxybenzoate 3-Carboxymuconate
Scheme 3.12 Biochemical pathway for the bioconversion of vanillin into 3-
carboxymuconate.
Another example of oxidative biocatalyzed cascade reactions fully performed in
vivo was presented by Liu and Li [34], who combined two metabolically nonrelated
enzymes by applying a real two-strain strategy. Specifically, a wild-type Acinetobacter
sp. strain and a recombinant E. coli strain expressing both a BVMO (CHMO Acineto )
and a glucose dehydrogenase were used to synthesize δ-hydroxycarboxylic acids
starting from α,β-unsaturated ketones. Owing to the lack of chemoselectivity – the
native enoate reductase NemA in the host E. coli interfered with the required
enoate reductase activity from the wild-type Acinetobacter strain – the whole reaction
sequence had to be performed stepwise. First, whole cells from Acinetobacter sp.
expressing a novel enoate reductase transformed the α,β-unsaturated substrate
into the corresponding saturated ketone. Only then the addition of the second
strain containing the second biocatalyst (CHMO Acineto ) together with a cofactor
regeneration cassette enabled the further oxygenation of the intermediate ketone
to the desired δ-lactones. Eventually, their hydrolysis to α,δ-hydroxycarboxylic acids
were catalyzed again by a hydrolase present in the wild-type Acinetobacter sp.
(Scheme 3.13).