Page 72 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 72
48 3 Monooxygenase-Catalyzed Redox Cascade Biotransformations
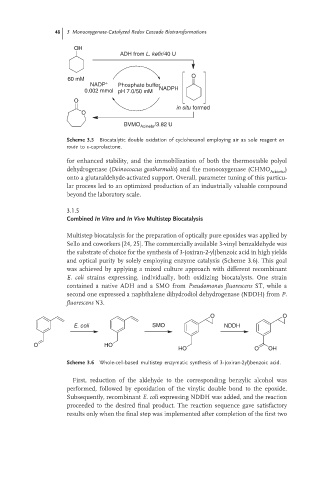
OH
ADH from L. kefir/40 U
O
60 mM
NADP + Phosphate buffer
0.002 mmol pH 7.0/50 mM NADPH
O
in situ formed
O
BVMO Acineto /3.82 U
Scheme 3.5 Biocatalytic double oxidation of cyclohexanol employing air as sole reagent en
route to ε-caprolactone.
for enhanced stability, and the immobilization of both the thermostable polyol
dehydrogenase (Deinococcus geothermalis) and the monooxygenase (CHMO )
Acineto
onto a glutaraldehyde-activated support. Overall, parameter tuning of this particu-
lar process led to an optimized production of an industrially valuable compound
beyond the laboratory scale.
3.1.5
Combined In Vitro and In Vivo Multistep Biocatalysis
Multistep biocatalysis for the preparation of optically pure epoxides was applied by
Sello and coworkers [24, 25]. The commercially available 3-vinyl benzaldehyde was
the substrate of choice for the synthesis of 3-(oxiran-2-yl)benzoic acid in high yields
and optical purity by solely employing enzyme catalysis (Scheme 3.6). This goal
was achieved by applying a mixed culture approach with different recombinant
E. coli strains expressing, individually, both oxidizing biocatalysts. One strain
contained a native ADH and a SMO from Pseudomonas fluorescens ST, while a
second one expressed a naphthalene dihydrodiol dehydrogenase (NDDH) from P.
fluorescens N3.
O O
E. coli SMO NDDH
O HO
HO O OH
Scheme 3.6 Whole-cell-based multistep enzymatic synthesis of 3-(oxiran-2yl)benzoic acid.
First, reduction of the aldehyde to the corresponding benzylic alcohol was
performed, followed by epoxidation of the vinylic double bond to the epoxide.
Subsequently, recombinant E. coli expressing NDDH was added, and the reaction
proceeded to the desired final product. The reaction sequence gave satisfactory
results only when the final step was implemented after completion of the first two