Page 74 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 74
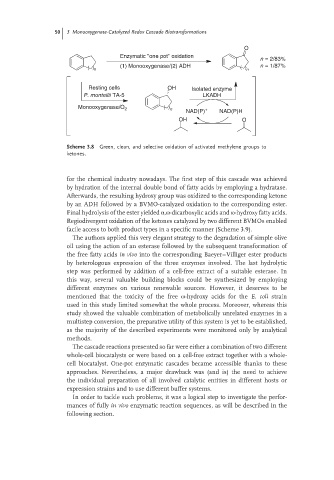
50 3 Monooxygenase-Catalyzed Redox Cascade Biotransformations
O
Enzymatic ''one pot'' oxidation
n = 2/83%
(1) Monooxygenase/(2) ADH n = 1/87%
n n
Resting cells OH Isolated enzyme
P. monteilii TA-5 LKADH
Monooxygenase/O 2 n +
NAD(P) NAD(P)H
OH O
Scheme 3.8 Green, clean, and selective oxidation of activated methylene groups to
ketones.
for the chemical industry nowadays. The first step of this cascade was achieved
by hydration of the internal double bond of fatty acids by employing a hydratase.
Afterwards, the resulting hydroxy group was oxidized to the corresponding ketone
by an ADH followed by a BVMO-catalyzed oxidation to the corresponding ester.
Final hydrolysis of the ester yielded α,ω-dicarboxylic acids and ω-hydroxy fatty acids.
Regiodivergent oxidation of the ketones catalyzed by two different BVMOs enabled
facile access to both product types in a specific manner (Scheme 3.9).
The authors applied this very elegant strategy to the degradation of simple olive
oil using the action of an esterase followed by the subsequent transformation of
the free fatty acids in vivo into the corresponding Baeyer–Villiger ester products
by heterologous expression of the three enzymes involved. The last hydrolytic
step was performed by addition of a cell-free extract of a suitable esterase. In
this way, several valuable building blocks could be synthesized by employing
different enzymes on various renewable sources. However, it deserves to be
mentioned that the toxicity of the free ω-hydroxy acids for the E. coli strain
used in this study limited somewhat the whole process. Moreover, whereas this
study showed the valuable combination of metabolically unrelated enzymes in a
multistep conversion, the preparative utility of this system is yet to be established,
as the majority of the described experiments were monitored only by analytical
methods.
The cascade reactions presented so far were either a combination of two different
whole-cell biocatalysts or were based on a cell-free extract together with a whole-
cell biocatalyst. One-pot enzymatic cascades became accessible thanks to these
approaches. Nevertheless, a major drawback was (and is) the need to achieve
the individual preparation of all involved catalytic entities in different hosts or
expression strains and to use different buffer systems.
In ordertotacklesuchproblems, it wasalogicalsteptoinvestigate theperfor-
mances of fully in vivo enzymatic reaction sequences, as will be described in the
following section.