Page 79 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 79
3.1 Introduction 55
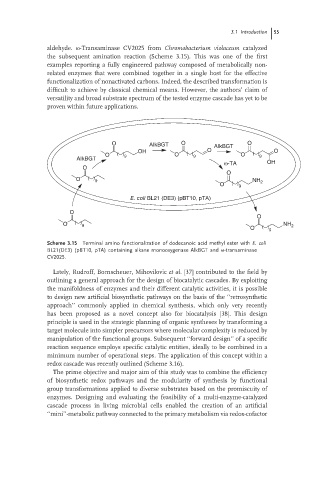
aldehyde. ω-Transaminase CV2025 from Chromobacterium violaceum catalyzed
the subsequent amination reaction (Scheme 3.15). This was one of the first
examples reporting a fully engineered pathway composed of metabolically non-
related enzymes that were combined together in a single host for the effective
functionalization of nonactivated carbons. Indeed, the described transformation is
difficult to achieve by classical chemical means. However, the authors’ claim of
versatility and broad substrate spectrum of the tested enzyme cascade has yet to be
proven within future applications.
O AIkBGT O AIkBGT O
OH O O
O 9 O 9 O 9
AIkBGT
ω-TA OH
O
O
O 9 NH
O 9 2
E. coli BL21 (DE3) (pBT10, pTA)
O
O
O 9 NH 2
O 9
Scheme 3.15 Terminal amino functionalization of dodecanoic acid methyl ester with E. coli
BL21(DE3) (pBT10, pTA) containing alkane monooxygenase AlkBGT and ω-transaminase
CV2025.
Lately, Rudroff, Bornscheuer, Mihovilovic et al. [37] contributed to the field by
outlining a general approach for the design of biocatalytic cascades. By exploiting
the manifoldness of enzymes and their different catalytic activities, it is possible
to design new artificial biosynthetic pathways on the basis of the ‘‘retrosynthetic
approach’’ commonly applied in chemical synthesis, which only very recently
has been proposed as a novel concept also for biocatalysis [38]. This design
principle is used in the strategic planning of organic syntheses by transforming a
target molecule into simpler precursors where molecular complexity is reduced by
manipulation of the functional groups. Subsequent ‘‘forward design’’ of a specific
reaction sequence employs specific catalytic entities, ideally to be combined in a
minimum number of operational steps. The application of this concept within a
redox cascade was recently outlined (Scheme 3.16).
The prime objective and major aim of this study was to combine the efficiency
of biosynthetic redox pathways and the modularity of synthesis by functional
group transformations applied to diverse substrates based on the promiscuity of
enzymes. Designing and evaluating the feasibility of a multi-enzyme-catalyzed
cascade process in living microbial cells enabled the creation of an artificial
‘‘mini’’-metabolic pathway connected to the primary metabolism via redox-cofactor