Page 78 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 78
54 3 Monooxygenase-Catalyzed Redox Cascade Biotransformations
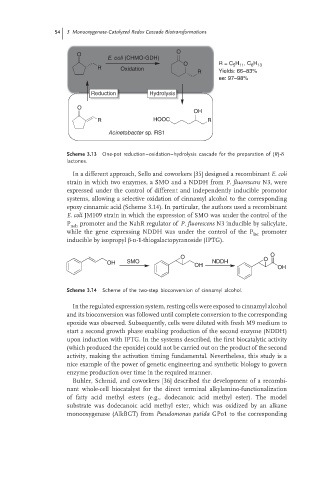
O
O
E. coli (CHMO-GDH)
H , C H
O R = C 5 11 6 13
R Oxidation
R Yields: 66–83%
ee: 97–98%
Reduction Hydrolysis
O
OH
R HOOC R
Acinetobacter sp. RS1
Scheme 3.13 One-pot reduction–oxidation–hydrolysis cascade for the preparation of (R)-δ
lactones.
In a different approach, Sello and coworkers [35] designed a recombinant E. coli
strain in which two enzymes, a SMO and a NDDH from P. fluorescens N3, were
expressed under the control of different and independently inducible promotor
systems, allowing a selective oxidation of cinnamyl alcohol to the corresponding
epoxy cinnamic acid (Scheme 3.14). In particular, the authors used a recombinant
E. coli JM109 strain in which the expression of SMO was under the control of the
P nah promoter and the NahR regulator of P. fluorescens N3 inducible by salicylate,
while the gene expressing NDDH was under the control of the P promoter
lac
inducible by isopropyl β-d-1-thiogalactopyranoside (IPTG).
O
O O
OH SMO OH NDDH
OH
Scheme 3.14 Scheme of the two-step bioconversion of cinnamyl alcohol.
In the regulated expression system, resting cells were exposed to cinnamyl alcohol
and its bioconversion was followed until complete conversion to the corresponding
epoxide was observed. Subsequently, cells were diluted with fresh M9 medium to
start a second growth phase enabling production of the second enzyme (NDDH)
upon induction with IPTG. In the systems described, the first biocatalytic activity
(which produced the epoxide) could not be carried out on the product of the second
activity, making the activation timing fundamental. Nevertheless, this study is a
nice example of the power of genetic engineering and synthetic biology to govern
enzyme production over time in the required manner.
Buhler, Schmid, and coworkers [36] described the development of a recombi-
nant whole-cell biocatalyst for the direct terminal alkylamino-functionalization
of fatty acid methyl esters (e.g., dodecanoic acid methyl ester). The model
substrate was dodecanoic acid methyl ester, which was oxidized by an alkane
monooxygenase (AlkBGT) from Pseudomonas putida GPo1 to the corresponding